People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Hunan Education Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
People's Education Press High School Chemistry Compulsory Course 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Cantonese Education Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"The Composition of Water" Source of Life - Water PPT Courseware
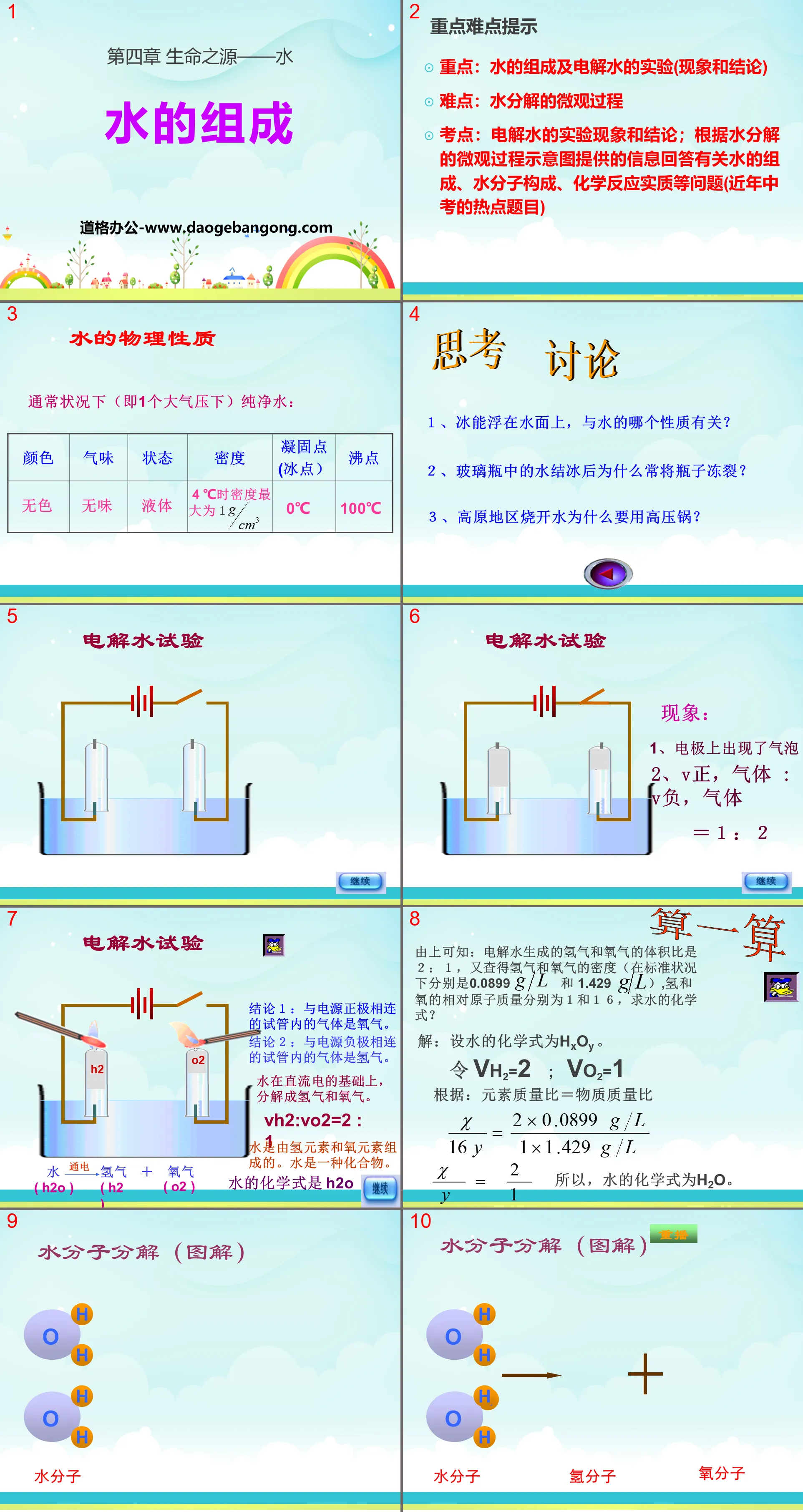
Important and difficult tips
Focus: The composition of water and the experiment of electrolyzing water (phenomenon and conclusion)
Difficulty: Microscopic process of water decomposition
Test points: Experimental phenomena and conclusions of electrolysis of water; answer questions about the composition of water, the composition of water molecules, and the nature of chemical reactions based on the information provided by the schematic diagram of the microscopic process of water decomposition (a hot topic in the high school entrance examination in recent years)
Think and discuss
1. The ability of ice to float on water is related to which property of water?
2. Why does the bottle often crack when the water in the glass bottle freezes?
3. Why do you need a pressure cooker to boil water in plateau areas?
Electrolyzed water test
Conclusion 1: The gas in the test tube connected to the positive electrode of the power supply is oxygen.
Conclusion 2: The gas in the test tube connected to the negative electrode of the power supply is hydrogen.
Water is decomposed into hydrogen and oxygen on the basis of direct current.
VH2:VO2=2:1
Water is composed of hydrogen and oxygen elements. Water is a chemical compound.
The chemical formula of water is H2O
Experimental results
macro aspect
Water is composed of two elements, hydrogen (H) and oxygen (O).
micro aspects
1. A water molecule is composed of 2 hydrogen atoms and 1 oxygen atom.
2. In chemical changes, molecules can be subdivided, but atoms cannot.
Response essence
When water molecules decompose, hydrogen atoms and oxygen atoms are generated. Two hydrogen atoms combine to form one hydrogen molecule, and many hydrogen molecules gather to form hydrogen gas; two oxygen atoms combine to form one oxygen molecule, and many oxygen molecules gather to form oxygen.
In a chemical reaction, molecules can be divided into atoms, but atoms cannot be divided further.
Application exercises
1. Among the following statements, which one is correct ( )
APure water is blue
BThe freezing point of water is 0℃ and the boiling point is 100℃
When C is 101kPa, the density of water at 4℃ is maximum
D Water can decompose into hydrogen and oxygen when boiled
2. Which of the following changes is different from the other three changes ( )
A water turns into ice B ice turns into water
C water turns into water vapor D water becomes electrified
3. When electrolyzing water, adding a small amount of sulfuric acid or sodium hydroxide to the water is ( )
APrevent accidentsBEnhance the conductivity of water
C gets more oxygen D gets more oxygen
Strengthening exercises:
1. Water is ( )
①Element; ②Mixture; ③Compound; ④Pure substance; ⑤Oxide
A. ①②③ B. ③④⑤ C. ①③⑤ D. ②③④⑤
2. Which of the following substances is a mixture ( )
A. Distilled water B. Water vapor C. Ice water D. Mineral water
3. Water is known as the "source of life". Which of the following properties of water are chemical properties ( )
A. Under normal circumstances, only about 30mL of oxygen can be dissolved in 1L of water
B. Water is a colorless and odorless gas
C. Water can generate hydrogen and oxygen under the action of electricity
D. Water can form ice when cooled to 0℃ at 1.01-105Pa
Extracurricular homework
1. Textbook exercises 1 and 2
2. How many grams of hydrogen are contained in 18 grams of water? How many grams of water contain 8 grams of oxygen?
Keywords: The source of life water teaching courseware, the composition of water teaching courseware, the Guangdong Education Edition ninth grade chemistry PPT courseware download, the ninth grade chemistry slide courseware download, the source of life water PPT courseware download, the composition of water PPT courseware download, .PPT format;
For more information about the PPT courseware "The Composition of Water, the Source of Life, Water", please click the "The Composition of Water, The Source of Life, Water ppt" tag.
"The Composition of Water" PPT courseware:
"The Composition of Water" PPT courseware The first part of the content: Think about it. Use the knowledge you have learned and combine it with the actual life to summarize the important physical properties of water. Thinking: Water changes during its circulation in nature. Is it a physical change or a chemical change? born..
"The Composition of Water" PPT:
"The Composition of Water" PPT Part One Content: Review: 1. Judgment of physical changes and chemical changes (1) Among the following phenomena, which one is a chemical change ( ) A. Glass breakage B. Tire leakage C. Gas burning D. Air liquefaction (2) The following processes belong to...
"The Composition of Water" Source of Life - Water PPT Courseware 2:
"The Composition of Water" PPT courseware of water, the source of life 2 1. Hydrogen 1. Physical properties: colorless, odorless gas, difficult to dissolve in water, less dense than air 2. Chemical properties: flammable hydrogen Purity test and combustion Observe the experiment carefully and record the experimental phenomena to verify the purity of hydrogen..
File Info
Update Time: 2024-10-21
This template belongs to Chemistry courseware Cantonese Education Edition Ninth Grade Chemistry Volume 1 industry PPT template
"The Composition of Water" Source of Life - Water PPT Courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "The Composition of Water" Source of Life - Water PPT Courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"The Composition of Water" Source of Life - Water PPT Courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview