"Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware 3 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Several Common Acids an... | 8025次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware 3 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware 3, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)

Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see








Authoritative PPT Summary
"Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware 3
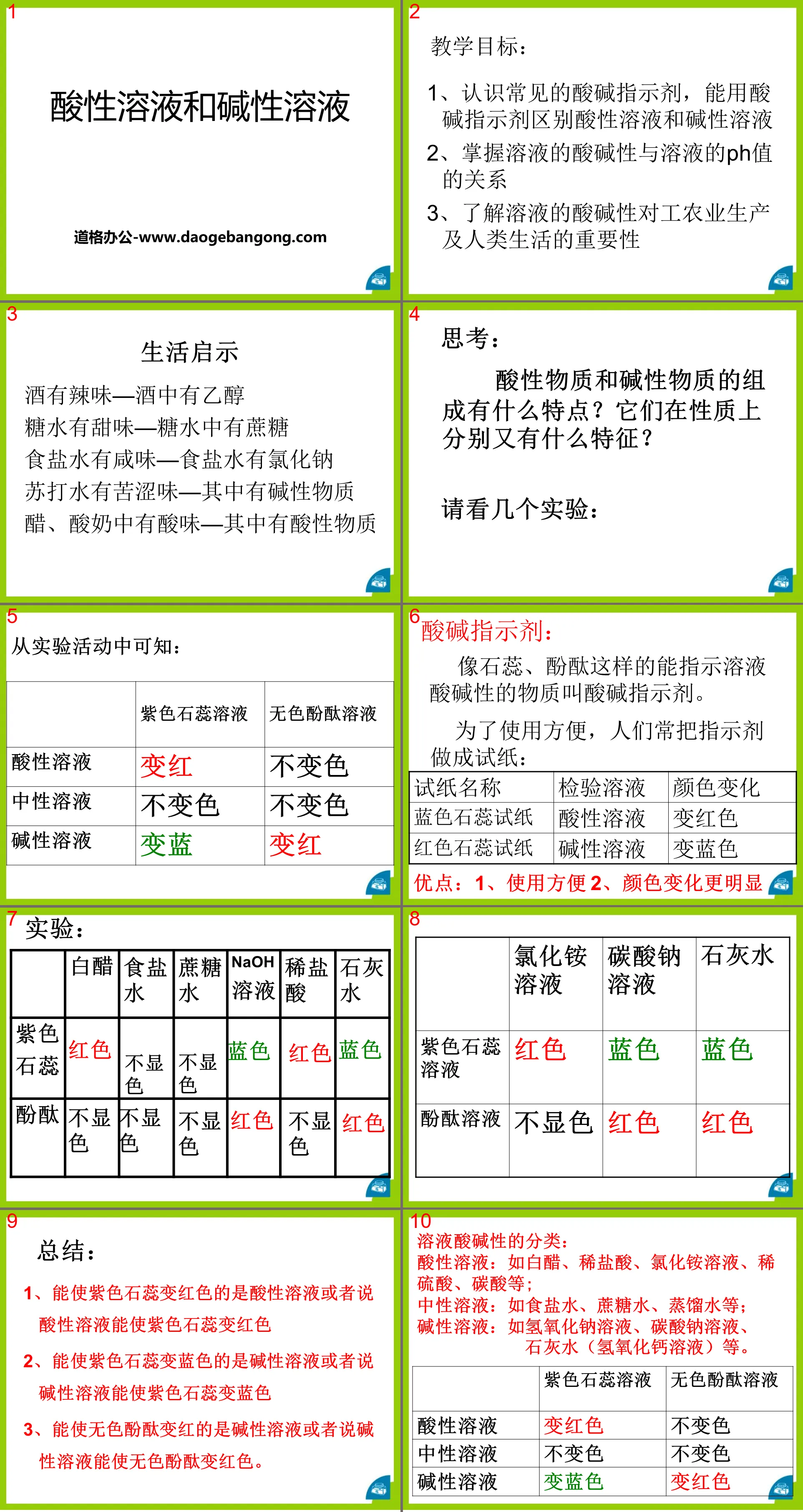
1. Several common acids:
1. Physical properties of concentrated hydrochloric acid and concentrated sulfuric acid
[Review questions]
1. What are acids and bases?
2. What are the composition characteristics of acids and bases?
Observe P11 Experiments 1, 2, and 3 and complete the following questions
Note: Because concentrated sulfuric acid has strong water absorption, it is often used as a desiccant for all gases except ammonia (NH3).
2. Dilution of concentrated sulfuric acid.
Observe P11 Experiment 4 and master the technique of diluting concentrated sulfuric acid.
Never pour water into concentrated sulfuric acid.
When diluting concentrated sulfuric acid, be sure to slowly pour the concentrated sulfuric acid into the water along the wall of the vessel and stir continuously.
The density of water is small and it floats on top of concentrated sulfuric acid. The heat released during dissolution will cause the water to boil immediately, causing the sulfuric acid droplets to splash around, which is very dangerous.
3. Overview of uses and dangers of hydrochloric acid (HCl)
(1) Hydrometallurgy for rare metals
(2) Used in organic synthesis
(3) Used in bleaching and dyeing industry
For example, hydrochloric acid is used for pickling after bleaching cotton cloth and neutralizing residual alkali after mercerizing cotton cloth. During the printing and dyeing process, some dyes are insoluble in water and need to be treated with hydrochloric acid to form soluble hydrochlorides before they can be used.
(4) Used in metal processing. For example, for pre-plating treatment of steel parts, first wash with caustic soda solution to remove oil stains, and then soak with hydrochloric acid; before metal welding, a little hydrochloric acid needs to be applied to the welding joint, etc., all of which are used Hydrochloric acid has the property of dissolving metal oxides to remove rust. In this way, the metal surface can be plated and welded firmly.
2. Several common bases
1. Sodium hydroxide (NaOH)
(1) Physical properties of sodium hydroxide
①Sodium hydroxide is a white solid.
②Sodium hydroxide is easily soluble in water and releases a lot of heat when dissolved.
③ Common names: fire soda, caustic soda, caustic soda.
(2)Characteristics of sodium hydroxide
①Corrosiveness:
Sodium hydroxide is highly corrosive. If you accidentally get lye on your skin, rinse with more water and then apply boric acid solution.
②Water absorption:
Sodium hydroxide easily absorbs moisture when exposed to the air, causing the surface to become moist and gradually dissolve. This phenomenon is called deliquescence.
Remember me: Because sodium hydroxide has strong water absorption, it is often used as a desiccant for hydrogen, oxygen, nitrogen, carbon monoxide, nitric oxide and other gases that cannot react with it. But it cannot be used to dry carbon dioxide, sulfur dioxide, and sulfur trioxide gases.
(3) The role of sodium hydroxide
2. Calcium hydroxide[ Ca(OH)2]
What reagent is used to detect carbon dioxide?
Lime water is an aqueous solution of calcium hydroxide.
Calcium hydroxide is commonly known as hydrated lime or hydrated lime. It can be obtained by the reaction of quicklime (CaO) and water:
CaO + H2O = Ca(OH)2 A large amount of heat is released during the reaction.
Calcium hydroxide is also corrosive to skin, clothing, etc.
Sodium hydroxide and calcium hydroxide are alkalis. In addition to these two alkali, commonly used alkali include potassium hydroxide (KOH), ammonia water (NH3·H2O), etc.
Class exercises
1. Which of the following descriptions about sodium hydroxide is incorrect ( )
A. Easily soluble in water and releases a lot of heat when dissolved
B. Strongly corrosive to skin
C. Aqueous solution can turn litmus solution red
D. Can remove oil stains and can be used as a kitchen cleaner
2. If you accidentally get lye on your skin, rinse with more _______ and then apply _______ solution.
3. Sodium hydroxide is highly corrosive, so its common names are _________, __________, and _________.
When it is exposed to the air, it is easy to _______________, and the surface becomes moist and gradually ___________. This phenomenon is called ____________; therefore, sodium hydroxide can be used as a _______________ for certain gases.
4. Clarified lime water can turn the purple litmus solution into ________ and the colorless phenolphthalein solution into ________. The solute in clarified lime water is ________, commonly known as ________ or __________.
Write the chemical equation that tests carbon dioxide ____________________________.
Keywords: Initial acids, bases and salts teaching courseware, several common acids and bases teaching courseware, Hunan Education Edition 9th grade chemistry PPT courseware download, Volume 2 chemistry download, 9th grade chemistry slide courseware download, Initial acids, bases and salts PPT courseware download, Download several common acids and bases PPT courseware, in .PPT format;
For more information about the PPT courseware "Initial acids, bases and salts, several common acids and bases", please click on the "Initial acids, bases and salts ppt, several common acids and bases" ppt tag.
"Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware 2:
"Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware 2 Preparation before class 1. Write the chemical formulas of common acids in life 2. Complete the following equations Mg Al Zn Fe; iron oxide, copper oxide and dilute respectively Chemical equation for the reaction of hydrochloric acid and dilute sulfuric acid. one..
"Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware:
"Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware Review before class 1. What kind of compound is called a base? Compounds in which all cations produced during ionization are H+ are called bases. 2. Which of the following compounds are bases? NaOH KOH Ca(OH)2 Ba(OH..