People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Cantonese Education Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
People's Education Press High School Chemistry Compulsory Course 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2 | pptx | 6 MB |
Description
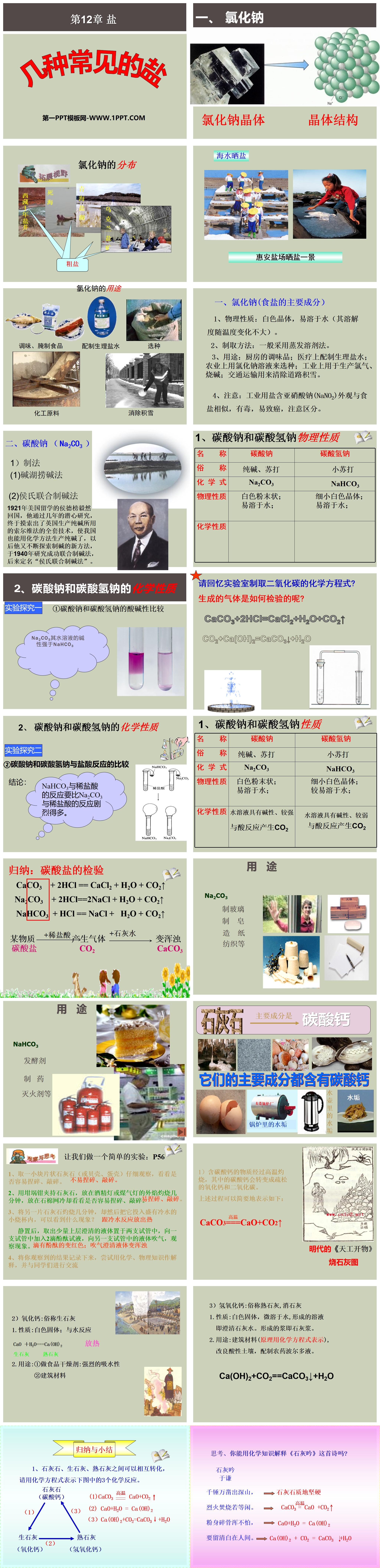
"Several Common Salts" Salt PPT Courseware
1. Sodium chloride (the main component of table salt)
1. Physical properties: white crystal, easily soluble in water (its solubility does not change much with temperature).
2. Preparation method: Generally, the solvent evaporation method is used.
3. Uses: kitchen condiments; medical preparation of physiological saline; agricultural use of sodium chloride solution for seed selection; industrial use for the production of chlorine and caustic soda; transportation use for clearing snow on roads.
4. Note: Industrial salt contains sodium nitrite (NaNO2), which looks similar to table salt and is toxic and easily carcinogenic. Pay attention to the distinction.
2. Sodium carbonate (Na2CO3)
1) Preparation method
(1) Alkali lake fishing method
(2) Hou's combined alkali production method
In 1921, Hou Debang, who studied in the United States, resolutely returned to China. After several years of painstaking research, he finally figured out the full set of Solvay process technology used in the production of soda ash in the United Kingdom, allowing my country to also produce soda ash using chemical methods. He continued to explore in the future. A new method of making alkali was successfully studied in 1940 and was later named "Hou's combined alkali making method".
1. Physical properties of sodium carbonate and sodium bicarbonate
2. Chemical properties of sodium carbonate and sodium bicarbonate
Experimental study one
① Comparison of the acidity and alkalinity of sodium carbonate and sodium bicarbonate
Experimental exploration two
②Comparison of the reactions of sodium carbonate and sodium bicarbonate with hydrochloric acid
Let's do a simple experiment: P56
1. Take a small piece of flaky limestone (or shell, eggshell) and observe it carefully to see if it is easy to crush or break.
2. Use crucible pliers to hold the limestone, place it in the outer flame of an alcohol lamp or gas lamp and burn it for a few minutes, then place it in an asbestos net to cool and see if it is easy to crush or break.
3. Burn another piece of limestone for a few minutes, then put it into a small beaker filled with cold water. What phenomena can be seen?
After letting it stand, take out a small amount of the upper clear liquid and place it in two test tubes. Add 2 drops of phenolphthalein test solution to one test tube, blow air into the liquid in the other test tube, and observe the phenomenon.
4. Record the results you observed, try to explain them with knowledge of chemistry and physics, and communicate with your classmates.
1) When materials containing calcium carbonate are burned at high temperatures, the calcium carbonate in them will be converted into loose calcium oxide and carbon dioxide.
The above process can be briefly expressed as follows:
CaCO3==CaO+CO2↑
2) Calcium oxide: commonly known as quicklime
1. Properties: white solid; reacts with water
CaO +H2O==Ca(OH)2 exothermic
Quick lime Hydrated lime
2.Usage:
① Use as food desiccant: strong water absorption
②Building materials
3) Calcium hydroxide: commonly known as slaked lime, slaked lime
1. Properties: White solid, slightly soluble in water, the resulting solution is clear lime water. The slurry formed is lime slurry.
2. Usage: building materials (the principle is represented by chemical equations), improving acidic soil, and preparing pesticide Bordeaux mixture.
Ca(OH)2+CO2==CaCO3↓+H2O
Summary and summary
1. Limestone, quicklime and hydrated lime can be converted into each other. Please use chemical equations to express the three chemical reactions in the figure below.
(1)CaCO3 = CaO+CO2
(2)CaO+H2O = Ca(OH)2
(3)Ca(OH)2+CO2=CaCO3↓+H2O
Thinking, can you use chemical knowledge to explain the poem "Song of Lime"?
lime chant
Yu Qian
Thousands of hammers were used to carve out the mountains, → the limestone is hard in texture
The fire burns as if it were idle. →CaCO3 = CaO +CO2
Don’t be afraid of being shattered into pieces, →CaO+H2O = Ca(OH)2
To remain innocent in this world. →Ca(OH)2 + CO2 = CaCO3+H2O
Keywords: salt teaching courseware, several common salt teaching courseware, Beijing curriculum reform version of the ninth grade chemistry PPT courseware download, ninth grade chemistry slide courseware download, salt PPT courseware download, several common salt PPT courseware download, .PPT format;
For more information about the PPT courseware "Salt: Several Common Salts", please click on the Salt ppt: Several Common Salts ppt tag.
"Several Common Salts" Salt PPT Courseware 3:
"Several Common Salts" Salt PPT Courseware 3 Knowledge Review Knowledge Point 1 Sodium Chloride 1. Sodium chloride is _________ crystal, ____ is soluble in water, has a salty taste, melting point is 801℃, boiling point is 1413℃, The solution is ____ and is the main component of _____. 2.Sodium chloride occurs naturally..
"Several Common Salts" Salt PPT Courseware 2:
"Several Common Salts" Salt PPT Courseware 2 Salt is the product of the neutralization of acid and alkali. It is formed by combining the acid anion in the acid and the metal cation in the base. Thinking: Is the salt we are talking about now the table salt we usually eat? It is not that salt is a class of substances that includes...
File Info
Update Time: 2024-09-09
This template belongs to Chemistry courseware Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2 industry PPT template
"Several Common Salts" Salt PPT Courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Several Common Salts" Salt PPT Courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Several Common Salts" Salt PPT Courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview