People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| People's Education Press High School Chemistry Compulsory Course I | pptx | 6 MB |
Description
"Periodic Table of Elements Nuclides" Atomic Structure and Periodic Table of Elements PPT Courseware
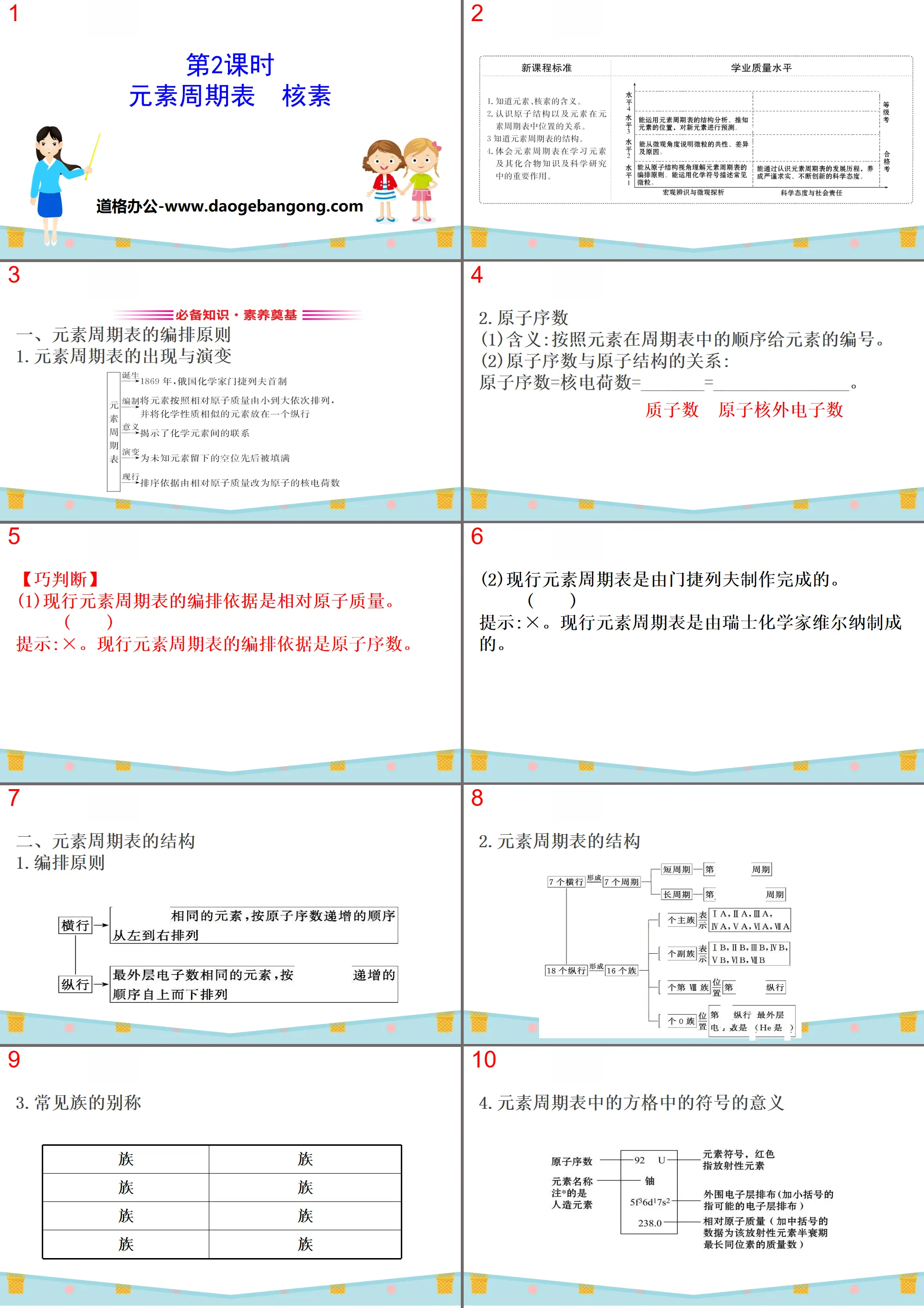
Part 1: Essential knowledge and foundation of literacy
1. Arrangement principles of the periodic table of elements
1. The emergence and evolution of the periodic table of elements
2.Atomic number
(1)Meaning: Number the elements according to their order in the periodic table.
(2)The relationship between atomic number and atomic structure:
Atomic number = nuclear charge = _______ = _______________.
【Smart Judgment】
(1) The current periodic table of elements is arranged based on relative atomic mass. ()
Tip:×. The current periodic table of elements is organized based on atomic numbers.
(2) The current periodic table of elements was produced by Mendeleev. ()
Tip:×. The current periodic table of elements was created by Swiss chemist Werner.
2. Structure of the periodic table of elements
1.Arrangement principles
2. Structure of the periodic table of elements
3. Nicknames for common tribes
4. The meaning of the symbols in the squares in the periodic table of elements
【Situation·Thinking】
In 1869, the Russian chemist Mendeleev designed and built a new home for the elements - the periodic table. Elements with the same number of electron shells were grouped on the same floor (same period), and a family with similar properties ( elements of the same family) are grouped into the same unit. You see, the elements are busy and happily moving into their new home!
(1) Which group of elements forms the most types of compounds?
Hint: Group IVA. The carbon element in Group IVA forms the largest variety of compounds.
(2) There are 2 electrons in the outermost layer of Fe, must they be in group IIA?
Tip: Elements in Group IIA must have 2 electrons in the outermost shell, but elements with 2 electrons in the outermost shell are not necessarily in Group IIA. Fe is a transition element.
3. Nuclides
1.Nuclide
An atom with a certain number of _____ and a certain number of _____ is called a nuclide. For example, 12C, 13C, and 14C are three different nuclides of carbon element.
2. Isotopes
(1) Definition: Different atoms of the same element that are _______ the same but _______ different are called isotopes. That is, different nuclides of the same element are called isotopes of each other. For example, the three nuclides H H and H are all isotopes of the hydrogen element.
(2) Example - isotopes of hydrogen element: 1H, 2H, 3H
(3) Characteristics of isotopes
①The chemical properties of various isotopes of the same element are slightly different. The physical properties are slightly different.
② In a naturally occurring element, whether in the free state or in the combined state, isotopes maintain a certain _____ with each other, that is, the _______________ occupied by various isotopes is the same.
【Micro thinking】
(1) Are all atoms composed of protons, neutrons, and electrons?
Hint: No. 1H atoms do not contain neutrons.
(2) What is the relationship between the type of nuclide and the number of protons, neutrons and mass number?
Periodic Table of Elements Nuclides PPT, Part 2: Key Competencies·Quality Formation
Knowledge point 1: The structure and simple application of the periodic table of elements
[Key points to clarify doubts]
1. Strengthen memory of the structure of the periodic table of elements
(1)Memory formula
The horizontal lines are called cycles, and there are currently one to seven, four are long and three are short, and the seventh is full. The vertical columns are called clans, and there are sixteen clans in total. One and eight appear one after another①, and one zero appears again②. There is one clan in one column, and clan VIII is special. Three verticals are counted as one clan, occupying 8, 9, and 10. Lanthanum series and actinium series, living in a small house is unsatisfactory, living in a crowded place at the age of fifteen, they both belong to the IIIB family.
Note: ① refers to ⅠA, ⅡA, ⅢB, ⅣB, ⅤB, ⅥB, ⅦB, Ⅷ;
② refers to ⅠB, ⅡB, ⅢA, ⅣA, ⅤA, ⅥA, ⅦA, 0.
(2) The relationship between column ordinal and family ordinal
① Column number <8, family number of main family and sub-family = column number;
② Column number = 8 or 9 or 10, which is Group VIII;
③ Column number > 10, family number of main family and sub-family = column number - 10 (except family 0).
(3) Transition elements
There are 10 vertical rows from IIIB to IIB in the periodic table of elements, including group VIII and all subgroup elements. There are more than 60 elements in total, all of which are metallic elements, collectively called transition elements.
2. Simple application of the periodic table of elements - element inference
(1) Use the relationship between the position of the element and the atomic structure to infer.
This method is commonly used to determine elements with atomic numbers less than 18.
①Application relationship.
Equation 1: Period number = number of electron shells
Equation 2: Main group number = number of outermost electrons
Equation 3: Atomic number = nuclear charge = number of protons = number of electrons outside the nucleus
②Example.
(�) The element
(�) The atomic number of element Y is 15, then the atomic structure diagram of this element is , and its position in the periodic table is Group VA of the third period.
3. Method to determine the position of an element based on its atomic number—noble gas positioning method
(1) Ratio of large and small determines the period
Compare the atomic number of the element with the number of the group 0 elements and find the group 0 elements adjacent to it. Then the element will be in the same period as the group 0 element with the larger number.
(2) Find the difference and determine the family number
① If the atomic number of an element is 1 or 2 more than that of the corresponding Group 0 element, the element should be in Group IA or Group IIA in the next cycle of the Group 0 element.
② If it is 1 to 5 less than the corresponding Group 0 element, it should be in Groups VIIA to IIIA of the same period.
③If the difference is another number, find the corresponding family based on the corresponding difference.
Knowledge point 2: The relationship between atomic structure and the number of particles. Comparison of the concepts of "four identicals"
[Key points to clarify doubts]
1. The constituent particles and functions of atoms
2. The number relationship between the constituent particles of atoms
(1)Electrically neutral atoms
(2) Calculation of the number of electrons in charged atoms-ions
3. Symbolic representation of particles
4. Differences and connections between elements, nuclides, isotopes and allotropes
【Think·Discussion】
(1) Do particles with the same number of protons definitely belong to the same element? Please give an example.
Tip: Not necessarily. Particles include atoms, simple anions and cations, atomic groups, molecules, etc. For example, H2O, CH4, Ne, etc. all have 10 protons, but they are not the same element.
(2) How to understand the word "isotope" in isotope?
Tip: Homogeny refers to occupying the same position in the periodic table of elements.
(3) Why are the number of types of elements much smaller than the number of types of atoms?
Tip: The same element can have many different isotope atoms, so the number of types of elements is much smaller than the number of types of atoms.
Keywords: PPT courseware for high school chemistry compulsory course 1 from the People's Education Press is available for free download, periodic table nuclide PPT download, atomic structure and periodic table PPT download, .PPT format;
For more information about the PPT courseware "Atomic Structure and Periodic Table of Elements and Periodic Table of Nuclides", please click on the Atomic Structure and Periodic Table of Elements ppt Periodic Table of Nuclides ppt tag.
"Atomic Structure and Periodic Table of Elements" Periodic Law of Material Structure PPT (Lesson 2 Atomic Structure and Properties of Elements):
"Atomic Structure and Periodic Table of Elements" Periodic Law of Material Structure Elements PPT (Lesson 2 Atomic Structure and Properties of Elements) Part One Content: Learning Objectives Course Standards 1. Understand the properties of alkali metal elements and halogen elements and their placement in the periodic table of elements relationship between the central position. ..
"Atomic Structure and Periodic Table of Elements" Periodic Law of Elements in Material Structure PPT (Lesson 1 Atomic Structure and Periodic Table of Elements Nuclides):
"Atomic Structure and Periodic Table of Elements" Periodic Law of Material Structure Elements PPT (Lesson 1 Atomic Structure Periodic Table Nuclides) Part One Content: Learning Objectives Course Standards 1. Understand the electron arrangement outside the nucleus. 2. Know the structure of the periodic table of elements. 3. Know..
"Atomic Structure and Properties of Elements" Atomic Structure and Periodic Table of Elements PPT Download:
"Atomic Structure and Properties of Elements" Atomic Structure and Periodic Table PPT Download Part One: Literacy Objectives 1. Know the atomic structure and characteristics of alkali metal elements and halogen elements through the atomic structure and atomic radius information in the textbook tables. 2. Through teaching materials..
File Info
Update Time: 2024-11-22
This template belongs to Chemistry courseware People's Education Press High School Chemistry Compulsory Course I industry PPT template
"Periodic Table of Elements Nuclides" Atomic Structure and Periodic Table of Elements PPT Courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Periodic Table of Elements Nuclides" Atomic Structure and Periodic Table of Elements PPT Courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Periodic Table of Elements Nuclides" Atomic Structure and Periodic Table of Elements PPT Courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview