"Periodic changes in the properties of elements" PPT download of the periodic law of elements Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Periodic changes in the... | 8500次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Periodic changes in the properties of elements" PPT download of the periodic law of elements is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Periodic changes in the properties of elements" PPT download of the periodic law of elements, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)

Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see








Authoritative PPT Summary
"Periodic changes in the properties of elements" PPT download of the periodic law of elements
Part One: Literacy Goals
1. Combine relevant data and experimental facts to understand the periodic change rules of the extranuclear electron configuration, main valence (the highest positive valence and the lowest negative valence), atomic radius and other properties of elements with the same period, and gradually construct the periodic law of elements.
2. Taking sodium, magnesium, aluminum, silicon, phosphorus, sulfur, and chlorine in the third period as examples, with the help of experimental exploration and combined with the knowledge of atomic structure, we can master the gradient law of metallicity and non-metallicity of main group elements in the same period, so as to Cultivate a sense of scientific inquiry and innovation.
3. Through the process of exploring the content and essence of the periodic law of elements, construct the research ideas and models that determine the macroscopic properties of microscopic atomic structure characteristics.
PPT on the periodic changes in the properties of elements, part 2: independent preview before class
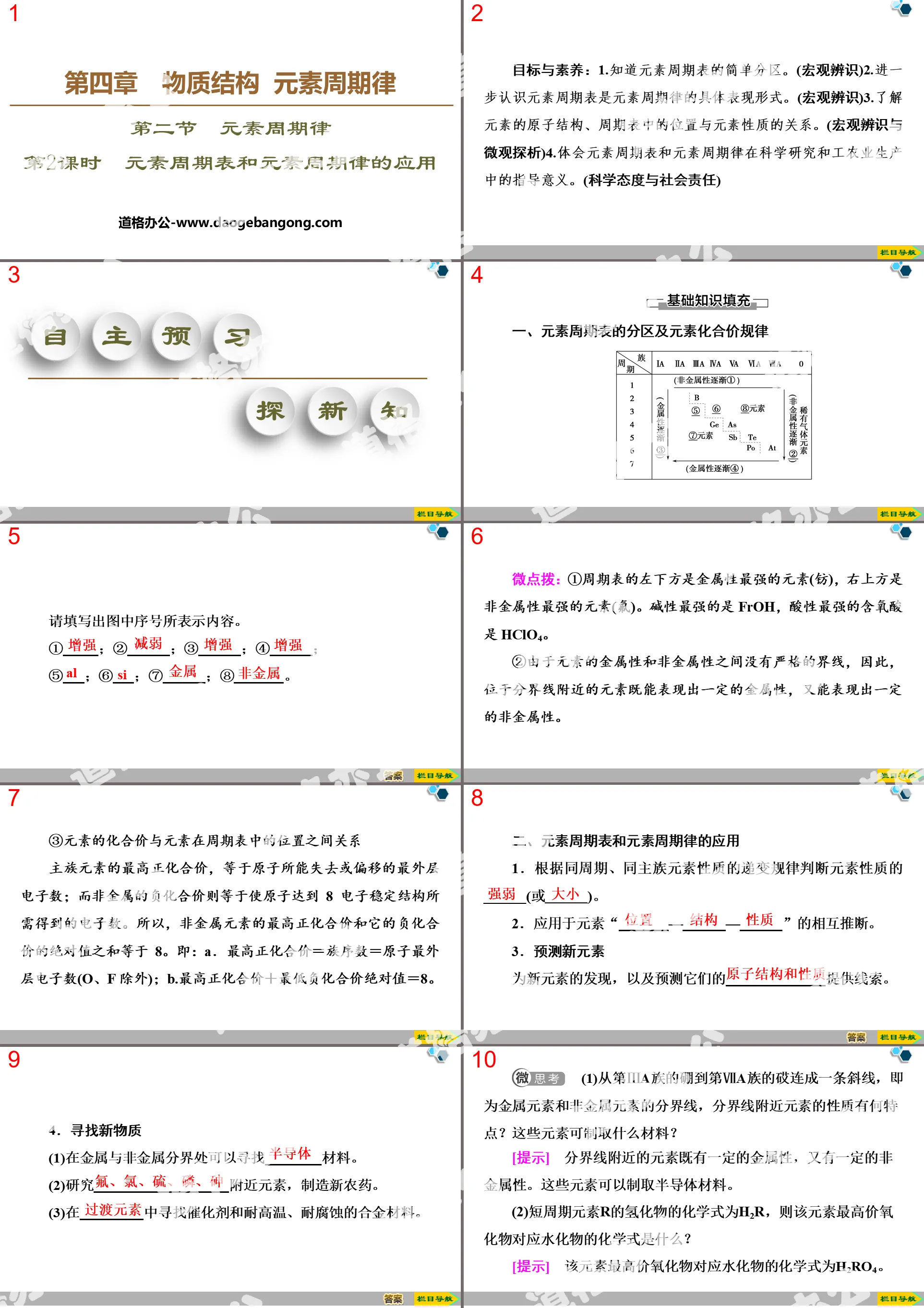
1. In the periodic table of elements, the names of the main group elements in the third period are sodium, magnesium, aluminum, silicon, phosphorus, sulfur, and chlorine.
2. The period number in the periodic table of elements is equal to the number of electron shells of the element atoms in that period, and the group number of the main group elements is equal to the number of electrons in the outermost shell of the atom. From top to bottom in the same main group, the metallicity gradually increases and the non-metallic property gradually weakens.
3. The atomic structure diagrams of magnesium, aluminum, sulfur and chlorine are respectively
Preview of new knowledge
1. Periodic changes in the properties of elements
1. Changes in the extranuclear electron configuration, atomic radius and main valence (the highest positive valence and the lowest negative valence) of elements 1 to 18
Conclusion: As the atomic number increases, the extranuclear electron arrangement, atomic radius and main valence of the element atoms all change periodically.
2. Gradient law of metallicity and non-metallicity of elements in the third period
(1) Comparison of metallic properties of Na, Mg and Al
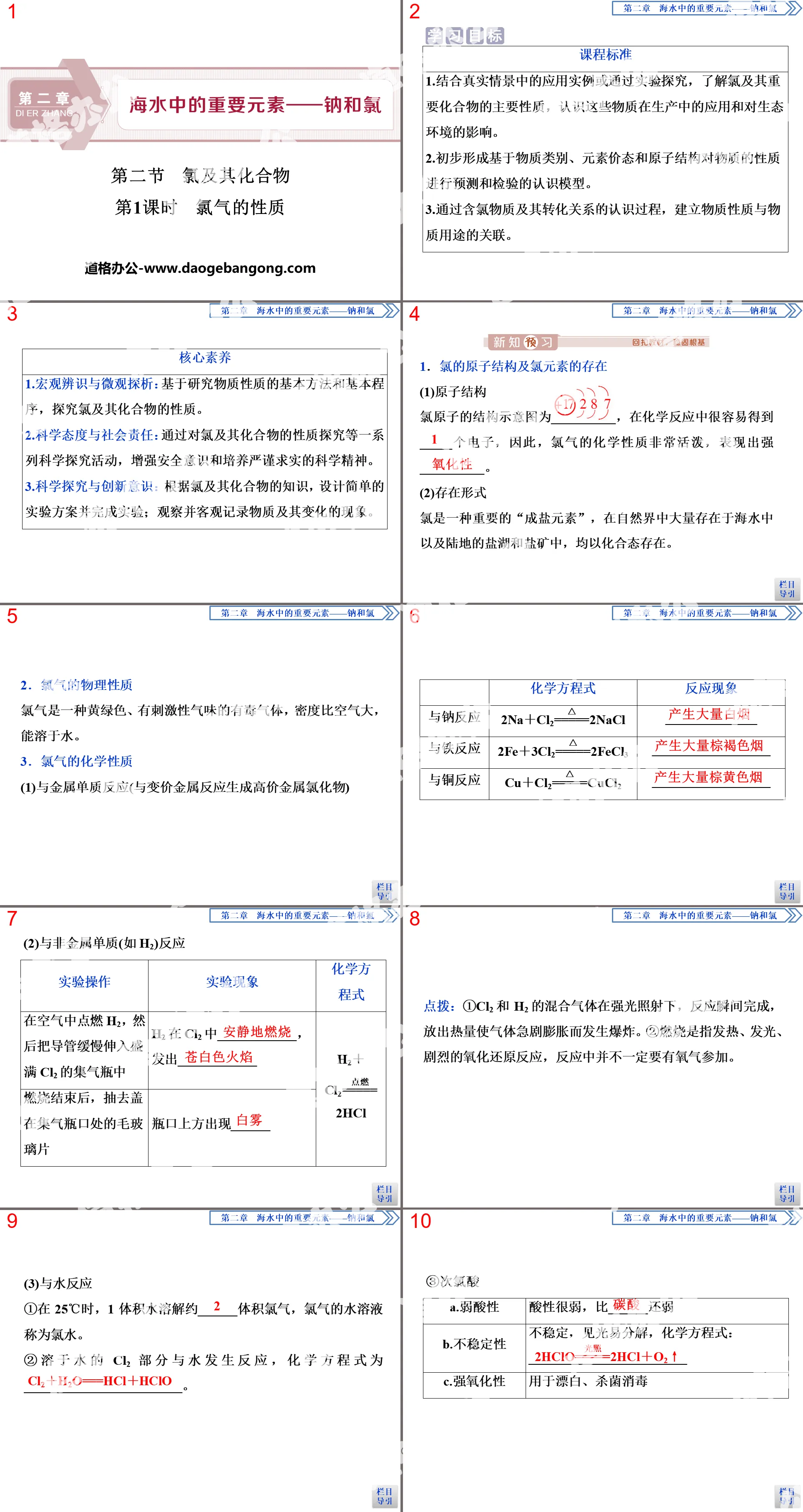
①Reacts with water
Sodium reacts violently with cold water to generate strong base NaOH and hydrogen, while releasing a large amount of heat. Magnesium reacts slowly with cold water. When the water boils, Mg can react with water to generate Mg(OH)2 and hydrogen.
[Micro Thought 1] Try to compare the state of water when Na, Mg, Fe and H2O react and whether heating is required?
It is suggested that Na reacts violently with cold water and releases a lot of heat; Mg reacts slowly with cold water but reacts violently with boiling water; Fe does not react with cold water or boiling water, but can react with water vapor at high temperatures.
②Generation and dissolution of the highest valence hydroxide
Al(OH)3:AlCl3+3NH3·H2O=Al(OH)3↓+3NH4Cl, Al(OH)3+NaOH=NaAlO2+2H2O.
Mg(OH)2:MgCl2+2NH3·H2O=Mg(OH)2↓+2NH4Cl, Mg(OH)2 is not dissolved in NaOH solution.
[Micro Thought 2] Al(OH)3 is the hydrate of Al2O3. How to generate Al(OH)3 from Al2O3?
It is suggested that Al2O3 and water cannot directly combine to form Al(OH)3. Al2O3 can be dissolved in dilute hydrochloric acid to obtain AlCl3, and then sufficient ammonia water can be added to the resulting solution to obtain Al(OH)3.
③The amphoteric properties of aluminum hydroxide
Aluminum hydroxide can react with acids to form salt and water, and can react with strong alkaline solutions to form salt and water. Al(OH)3 is an amphoteric hydroxide. The ionic equations of the reactions are Al(OH)3+3H+=Al3++3H2O and Al(OH)3+OH-=Al +2H2O.
Conclusion: NaOH is a strong base, Mg(OH)2 is a medium-strong base, Al(OH)3 is an amphoteric hydroxide, and the metallicity of Na, Mg, and Al gradually weakens.
(2) Comparison of non-metallic properties of Si, P, S and Cl
Conclusion: The non-metallic properties of Si, P, S and Cl gradually increase.
(3) Gradient law of metallicity and non-metallicity of elements in the third period
2. Periodic law of elements
1. Content: The properties of elements change periodically as the atomic number increases.
2. Essence: Periodic changes in the properties of elements are the inevitable result of periodic changes in the electron configuration outside the nucleus of atoms.
Autonomous testing
1. Judge whether it is right or wrong (mark “√” if it is correct and “×” if it is wrong).
(1) For elements of the same period, from left to right, the atomic radius gradually decreases, and the ionic radius also gradually decreases. ()
(2) In the second period, the elements go from left to right, and the highest positive price increases from +1 to +7. ()
(3) The more electrons an element gains, the more non-metallic it is; the more electrons it loses, the more metallic it is. ()
(4) Al(OH)3 is an amphoteric hydroxide and can react with ammonia and hydrochloric acid. ()
(5) Adding sufficient ammonia water to the AlCl3 solution can form Al(OH)3 precipitation. ()
(6) The stronger the acidity of the hydrate of an element's oxide, the stronger the non-metallic property; the stronger the alkalinity, the stronger the metallic property. ()
(7) After the gaseous hydrides of non-metallic elements in the second period are dissolved in water, the aqueous solutions are all acidic. ()
Answer(1)× (2)× (3)× (4)× (5)√ (6)× (7)×
2. Compare the following sets of properties according to the periodic law of elements.
(1) Metallicity: K_______Na_______Mg,
Non-metallic: F_______O_______S.
(2) Alkaline: Mg(OH)2_______Ca(OH)2_______KOH.
(3) Acidity: HClO4_______H2SO4_______HClO.
(4) Thermal stability: CH4_______NH3_______H2O.
Answer(1)>>(2)<<(3)>> (4)<<
PPT on the periodic changes in the properties of elements, part three: classroom exploration and learning
Periodic changes in the properties of elements with the same period
Question exploration
Consider the following questions using the third period elements as an example:
1. Comparison of the metallicity of Na, Mg and Al
(1) Basis
① When Na, Mg, and Al replace hydrogen in water (or acid), the order from easy to difficult is___________;
②The order of alkalinity from strong to weak of the hydrates of the highest-priced oxides of Na, Mg, and Al is___________.
(2) Conclusion: The order of metallicity from strong to weak of sodium, magnesium and aluminum is ___________.
Tips (1)Na, Mg, Al NaOH>Mg(OH)2>Al(OH)3 (2)Na>Mg>Al
2. Comparison of the non-metallic properties of Si, P, S and Cl
(1) Basis
①The order of conditions from easy to difficult when the simple substances of Si, P, S, and Cl are combined with H2 is___________;
②The order of acidity from strong to weak in the hydrates of the highest-priced oxides of Si, P, S, and Cl is _______________________.
(2) Conclusion: The order of non-metallic properties of Si, P, S and Cl from strong to weak is ___________.
Tips (1)①Cl, S, P, Si ②HClO4>H2SO4>H3PO4>H2SiO3 (2)Cl>S>P>Si
3. The law of gradual change of properties of elements in the same period
PPT on the periodic changes in the properties of elements, part 4: in-class testing
1. Using aluminum oxide as raw material to prepare aluminum hydroxide, the best method is ()
A. Dissolve aluminum oxide in water
B. Dissolve the aluminum oxide in hydrochloric acid first, then add ammonia dropwise
C. Dissolve aluminum oxide in hydrochloric acid, then add sodium hydroxide solution dropwise
D. Dissolve aluminum oxide in potassium hydroxide
Answer B
Analysis: To prepare aluminum hydroxide from aluminum oxide, the aluminum oxide should first be converted into aluminum ions, and then alkali should be added to convert it into aluminum hydroxide. Considering that when sodium hydroxide solution is added dropwise, part of the aluminum hydroxide will be converted into metaaluminate if it is excessive, so it is better to use a weak alkali solution of ammonia water.
2. Which of the following gradual changes in the properties of elements is wrong ()
A.The number of electrons in the outermost shell of Li, Be, and B atoms increases in sequence.
The highest positive valence of elements B.P, S, and Cl increases in sequence.
C. The number of electron layers of Na, K, and Rb increases in sequence.
D.N, O, and F atomic radii increase in sequence.
AnswerD
Analyze this question to examine the law of gradients in the properties of elements. As the atomic number increases, the atomic structure, atomic radius, metallicity and non-metallicity of elements change periodically. All three items A, B, and C are correct; item D The atomic radius should decrease successively.
3. The following groups of particles are arranged in order from largest to smallest radius. Which one is correct ()
A.Mg, Ca, K, Na B.S2-, Cl-, K+, Na+
C.Br-, Br, Cl, S D.Na+, Al3+, Cl-, F-
Answer B
Analysis: K and Ca have one more electron shell than Na and Mg, so r(K)>r(Ca)>r(Na)>r(Mg), item A is wrong; S2-, Cl-, K+ three ion nuclei The external electron arrangement is the same. The smaller the nuclear charge, the larger the ionic radius. And because K+ has one more electron shell than Na+, r(S2-)>r(Cl-)>r(K+)>r(Na+ ), item B is correct; Br- has one more electron than Br and has a larger radius. Br has one more electron shell than Cl, so r(Br-)>r(Br)>r(Cl), but r(Cl)< r(S), item C is wrong; the electron arrangement outside the nucleus of Na+, Al3+, and F- is the same. The smaller the nuclear charge, the larger the ionic radius. Cl- has one more electron shell than F-, so there is r(Cl- )>r(F-)>r(Na+)>r(Al3+), item D is wrong.
Keywords: PPT courseware for high school chemistry compulsory course 1 from the People's Education Press is available for free download, PPT download on the periodic changes in the properties of elements, PPT download on the periodic law of elements, .PPT format;
For more information about the PPT courseware "Periodic Law of Elements and the Periodic Changes in the Properties of Elements", please click the "Periodic Law of Elements ppt Periodic Changes in the Properties of Elements" ppt tag.
"Inference of Elements" Periodic Law of Elements in Material Structure PPT:
"Inference of Elements" Material Structure Elements Periodic Law PPT Part One Content: Knowledge, Ability and Intensive Lecture Element inference questions occupy a certain proportion in the college entrance examination over the years (mainly reflected in multiple-choice questions), mainly testing the position, structure, and sex of the periodic table of elements Relationships and the use of material structures and...
"End of Chapter Review Lesson" Periodic Law of Elements in Material Structure PPT:
"End of Chapter Review Course" Periodic Law of Elements in Material Structure PPT Special Topic Summary Practice two important methods for inferring the position of elements in the periodic table 1. Infer the periodic number and group number from the atomic number. For elements with a long period, subtract the atomic number from the Smaller and closer rare gas...
"End-of-Chapter Integration Improvement" Periodic Law of Material Structure Elements PPT:
"End-of-Chapter Integration Improvement" Periodic Law of Material Structure Elements PPT Part One Content: 1. Judgment of the metallicity and non-metallicity of elements 1. Judgment of metallicity (1) Judgment based on the periodic table of elements ①In the same period, from left to right, the metallicity of elements gradually decreases..