People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Hunan Education Edition Ninth Grade Chemistry Volume 1
Cantonese Education Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 2
People's Education Press High School Chemistry Compulsory Course 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Hunan Education Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"Particles constituting matter" The composition of matter PPT courseware 2
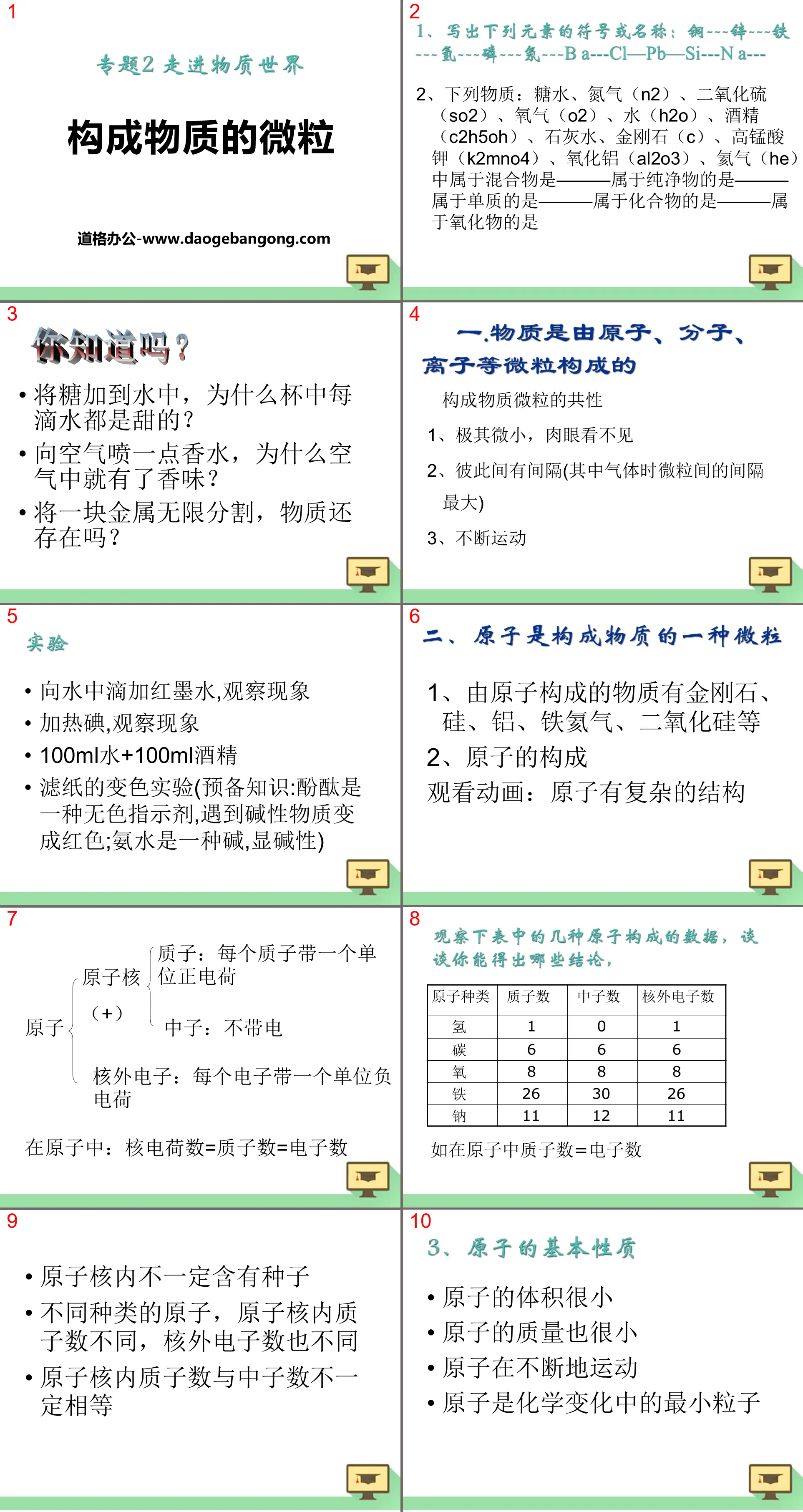
do you know?
When sugar is added to water, why does every drop of water in the cup taste sweet?
Spray a little perfume into the air. Why is there a fragrance in the air?
If a piece of metal is divided infinitely, will matter still exist?
1. Matter is composed of atoms, molecules, ions and other particles
Common characteristics of the particles that make up matter
1. Extremely tiny and invisible to the naked eye
2. There is a gap between each other (the gap between particles is the largest when it is a gas)
3. Keep moving
Add drops of red ink to the water and observe the phenomenon
Heat iodine and observe the phenomenon
100ml water + 100ml alcohol
Discoloration experiment of filter paper (preliminary knowledge: phenolphthalein is a colorless indicator, which turns red when encountering alkaline substances; ammonia is an alkali, showing alkalinity)
2. Atom is a kind of particle that makes up matter.
1. Substances made of atoms include diamond, silicon, aluminum, iron, helium, silica, etc.
2. The composition of atoms
Watch animation: Atoms have complex structures
Nucleus(+)
Proton: Each proton carries one unit of positive charge
Neutron: uncharged
Extranuclear electrons: each electron carries a unit negative charge
In an atom: nuclear charge = number of protons = number of electrons
There may not necessarily be seeds in the nucleus
Different types of atoms have different numbers of protons in the nucleus and different numbers of electrons outside the nucleus.
The number of protons and neutrons in the nucleus are not necessarily equal
3. Elements
1. The concept of element: a general term for atoms of the same type with the same nuclear charge.
What determines the type of an element is the nuclear charge (number of protons); elements only talk about the type but not the number, while atoms talk about both the number and the type.
2. The meaning of element symbols
1. Represents an element
2. Represents an atom of this element
3. Indicates the relative atomic mass of the element’s atoms
The meaning of N: ① represents nitrogen element ② represents a nitrogen atom ③ represents the relative atomic mass of nitrogen atom is about 14
The meaning of 2N: 2 nitrogen atoms
When there are numbers before element symbols, the macro meaning is lost and only the micro meaning is lost.
4. Molecules are also particles that make up matter.
1. The substances composed of molecules are: nitrogen (N2) oxygen (O2) water (H2O) sulfur dioxide (SO2) alcohol (C2H5OH)
2. Molecules are composed of atoms
For example: Each water molecule (H2O) consists of 2 hydrogen atoms and 1 oxygen atom
3. Basic properties of molecules
①The molecules are very small;
②There are gaps between molecules;
③ Molecules are constantly moving;
④ Molecules are the smallest particles that maintain the chemical properties of substances; the same kind of molecules have the same properties, and different kinds of molecules have different properties.
Keywords: teaching courseware of the composition of matter, teaching courseware of particles that make up matter, download the Chemistry PPT courseware for the first volume of the ninth grade of the Hunan Education Edition, download the chemistry slide courseware for the ninth grade, download the PPT courseware of the composition of matter, download the PPT courseware of the particles that make up a substance, .PPT format;
For more information about the PPT courseware "The composition of matter and the particles that make up matter", please click the "The composition of matter ppt and the particles that make up matter" ppt tag.
"Molecular" PPT courseware of particles that make up matter:
"Molecular" PPT courseware of particles constituting matter. Can you clearly explain the following phenomena? 1. The perfume is not sprayed on your nose, so why can you smell the pungent fragrance? 2. Why do we often smell the fragrance of flowers or wine near gardens or hotels? 3. Sheng..
"Electron Configuration Outside the Atomic Nucleus" PPT courseware of particles constituting matter:
"Arrangement of Electrons Outside the Atomic Nucleus" PPT courseware of particles constituting matter 1. Arrangement of electrons outside the atomic nucleus (layered arrangement) 1. The relationship between the energy of electrons and their distance from the nucleus: One, two, three, four, five, six, seven electrons Layer K L M N O P Q Far, near, far from the core..
"Atoms" PPT courseware 2 of the particles that make up matter:
"Atoms" The particles that make up matter PPT courseware 2 1. Preliminary understanding of atoms Characteristics of atoms: Atoms are small, have mass, and have volume. How much is the mass of an atom? The mass of a carbon atom is: 0.00000000000000000000000001993kg, which is 1.993..
File Info
Update Time: 2024-10-20
This template belongs to Chemistry courseware Hunan Education Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Particles constituting matter" The composition of matter PPT courseware 2 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Particles constituting matter" The composition of matter PPT courseware 2 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Particles constituting matter" The composition of matter PPT courseware 2, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview