"Oxygen" The air around us PPT courseware 4 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Oxygen" The air around... | 23650次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Oxygen" The air around us PPT courseware 4 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Oxygen" The air around us PPT courseware 4, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see










Authoritative PPT Summary
"Oxygen" The air around us PPT courseware 4
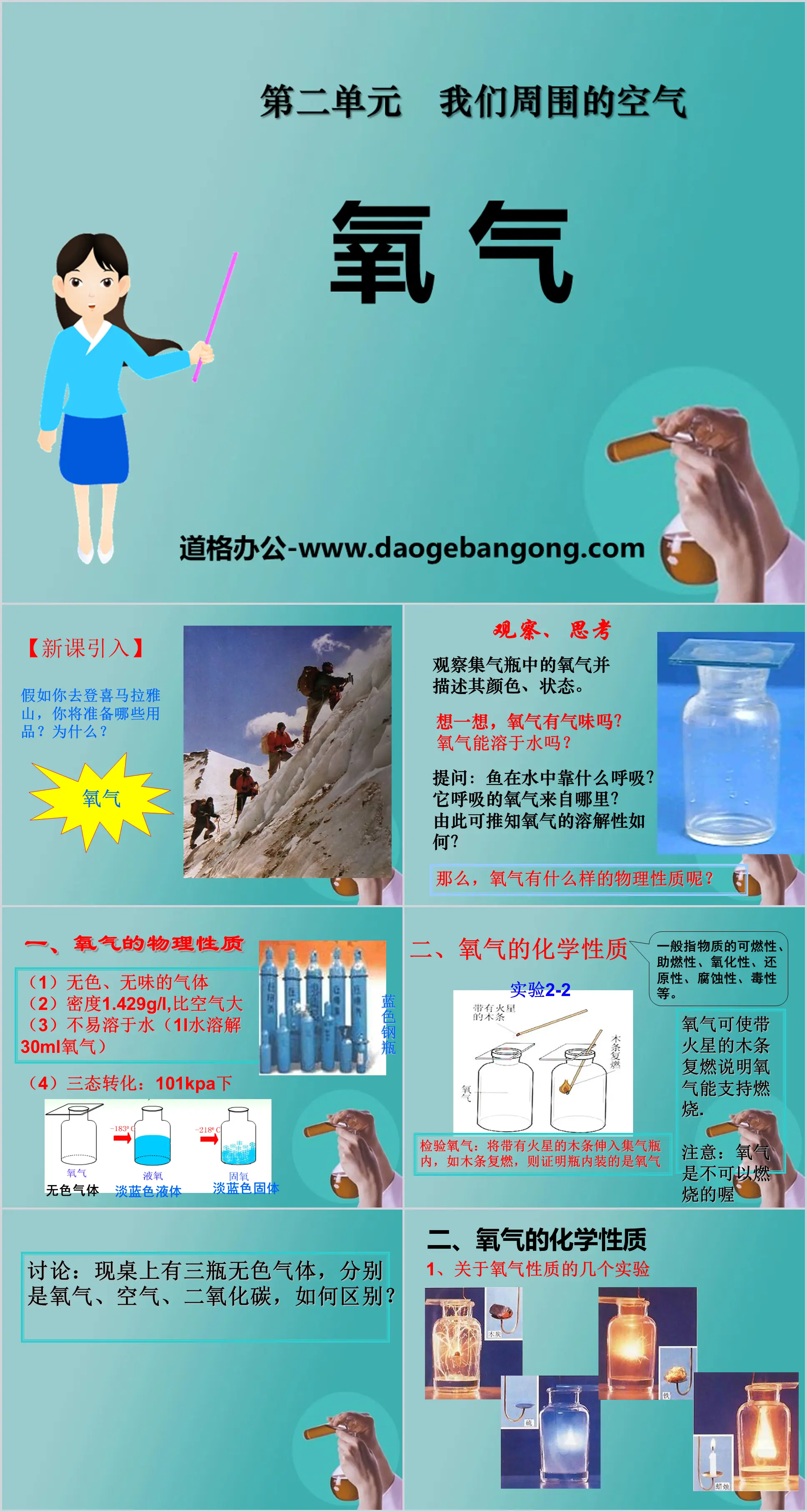
observe, think
Observe the oxygen in the gas cylinder and describe its color and state.
Think about it, does oxygen smell?
Can oxygen dissolve in water?
Question: How do fish breathe in water? Where does the oxygen it breathes come from?
What can be inferred from this about the solubility of oxygen?
1. Physical properties of oxygen
(1) Colorless, odorless gas
(2) Density 1.429g/L, larger than air
(3) Not easily soluble in water (1L of water dissolves 30mL of oxygen)
(4) Three-state transformation: under 101kPa
2. Chemical Properties of Oxygen
Generally refers to the flammability, combustion-supporting property, oxidizing property, reducing property, corrosiveness, toxicity, etc. of the substance.
Oxygen can rekindle wooden sticks with sparks, indicating that oxygen can support combustion.
Note: Oxygen cannot be burned.
Test oxygen: Insert the wooden stick with sparks into the gas collecting bottle. If the wooden stick re-ignites, it proves that the bottle contains oxygen.
do you know?
Things to note for a successful experiment:
①The function of matches: to ignite the iron wire
② Wrap the wire into a spiral: increase the contact area with oxygen
③When the match is about to burn out, put the iron wire into the oxygen bottle: to prevent the match from consuming too much oxygen and preventing the iron wire from burning smoothly.
④Put a small amount of water in the gas collecting bottle in advance or spread a thin layer of fine sand at the bottom of the bottle to prevent high-temperature melt from splashing down and exploding the bottom of the bottle.
⑤Use sandpaper to remove the rust on the wire surface
Determination of oxygen content in air
When doing this experiment, would it be okay to choose sulfur, carbon, candle, iron or magnesium as the drug?
① Sulfur, carbon, and candles cannot be selected: because they burn in the air, consume oxygen and generate new gases, they cannot form a pressure difference, and water cannot be sucked back.
② Iron or aluminum cannot be selected: because they cannot burn in air.
③ Magnesium cannot be selected: because it burns in the air and consumes oxygen, and it can also react with nitrogen and carbon dioxide in the air, which will cause far more than one-fifth of the water to enter.
Drug selection: It can burn in the air, and only reacts with oxygen, and the product is gas-free (red phosphorus is the most suitable).
Targeted exercises
1. Which of the following descriptions about the properties of oxygen is incorrect ( )
A. Under normal circumstances, oxygen is a colorless and odorless gas
B. Oxygen can become liquid or solid at low temperature and high pressure
C. Oxygen is easily soluble in water
D. Oxygen is a gas with relatively active chemical properties.
2. Which of the following statements is correct ( )
A. Charcoal produces black solids after burning
B. The wire is inserted into a gas bottle containing oxygen and burns violently
C. Red phosphorus cannot burn in air
D. Sulfur generates pungent smelling gas after burning.
3. Among the following properties of substances, which ones are chemical properties ( )
A. Color, state B. Density, hardness
C. Oxidizing, flammable D. Melting point, boiling point
4. The phenomenon that must occur when a substance burns in air is ( )
A. Generate solid substances B. Generate gas substances
C. Release heat D. Emit white light
5. Among the following substances, which one does not contain oxygen ( )
A.Carbon dioxide B.Natural water
C. Clean air D. Liquid oxygen
5. Slow oxidation:
An oxidation reaction that proceeds very slowly or even imperceptibly is called slow oxidation.
For example: the respiration of animals and plants, the decay of food, the brewing of wine and vinegar, the decay of farmyard manure, the rust of iron and copper
6. Spontaneous combustion:
Spontaneous combustion caused by slow oxidation.
For example: white phosphorus spontaneous combustion, straw spontaneous combustion, etc.
Not all slow oxidation will trigger spontaneous combustion.
General conclusion: Oxygen is a gas with relatively active chemical properties. It provides oxygen in oxidation reactions, has oxidizing properties, and is a commonly used oxidizing agent.
Keywords: Air around us teaching courseware, Oxygen teaching courseware, People's Education Edition ninth grade chemistry PPT courseware download, Ninth grade chemistry slide courseware download, Air around us PPT courseware download, Oxygen PPT courseware download, .PPT format;
For more information about the PPT courseware "Oxygen in the Air Around Us", please click the Oxygen in the Air Around Us ppt tag.
"Air and Oxygen" PPT download:
"Air and Oxygen" PPT Download Part One: Preparing Oxygen in the Laboratory 1. Steps to produce oxygen using hydrogen peroxide (hydrogen peroxide): 1. Check the air tightness of the device; 2. Add MnO2 to the flask; 3. Connect the device; 4. Add H2O2 to the separatory funnel; 5..
"Air and Oxygen" PPT courseware:
"Air and Oxygen" PPT courseware Part 1: Composition of air 1. Prove that the air contains carbon dioxide: -----Air can make clear lime water turbid. 2. Proof that there is water vapor in the air: -----When ice is placed in a cup, water droplets appear on the wall of the cup. -----Anhydrous sulfur..
"Air and Oxygen" PPT:
"Air and Oxygen" PPT Part One: Properties of Oxygen 1. Observe the color and state of a bottle of oxygen. 2. Gently fan the mouth of the bottle with your hand to let a small amount of oxygen float into your nostrils and smell its smell. Oxygen is a ____color____odorous gas. Smell...