"Oxygen" air and water PPT courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Oxygen" air and water P... | 7125次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Oxygen" air and water PPT courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Oxygen" air and water PPT courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)

Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see







Authoritative PPT Summary
"Oxygen" air and water PPT courseware
1. Properties of Oxygen
1. Physical properties of oxygen
(1) Normally, colorless and odorless gas
(2) The density is slightly greater than that of air
(3) Not easily soluble in water
Oxygen test
(1) Oxygen can rekindle wood sticks with sparks. (That is, oxygen can support combustion)
Check oxygen:
Insert the wooden stick with sparks into the gas collecting bottle. If the wooden stick ignites again, it proves that the bottle contains oxygen.
chemical properties of oxygen
(1) Reaction of sulfur and oxygen
Sulfur burns in the air
【Phenomenon】
1. Light blue flame 2. Heat release 3. Generate pungent smelling gas
Sulfur burns in oxygen
【Phenomenon】
1. Blue-purple flame 2. Heat release 3. Generate pungent smelling gas
(2) Reaction of carbon and oxygen
Carbon burns in the air
【Phenomenon】
1. Redness 2. Heat release 3. The generated gas can turn clear lime water into turbidity
Carbon burns in oxygen
【Phenomenon】
1. White light 2. Heat release 3. The generated gas can turn clear lime water into turbidity
(3) Reaction of iron and oxygen
【Experimental phenomena】
1. Burn violently, with sparks shooting out. 2. Release a lot of heat. 3. Produce black solids.
[Thinking] Why put some water or spread a layer of fine sand at the bottom of the bottle before the experiment?
discuss:
1. Why is the wire coiled in a spiral shape?
Increase the heating area of the iron wire. Increase the heating area of the iron wire.
2. What is the purpose of tying a match to the lower end? Ignite the wire.
3. Why wait until the match is almost burned out before putting it into the bottle?
Prevent matches from burning and consuming oxygen in the bottle.
2. Combination reactions and oxidation reactions
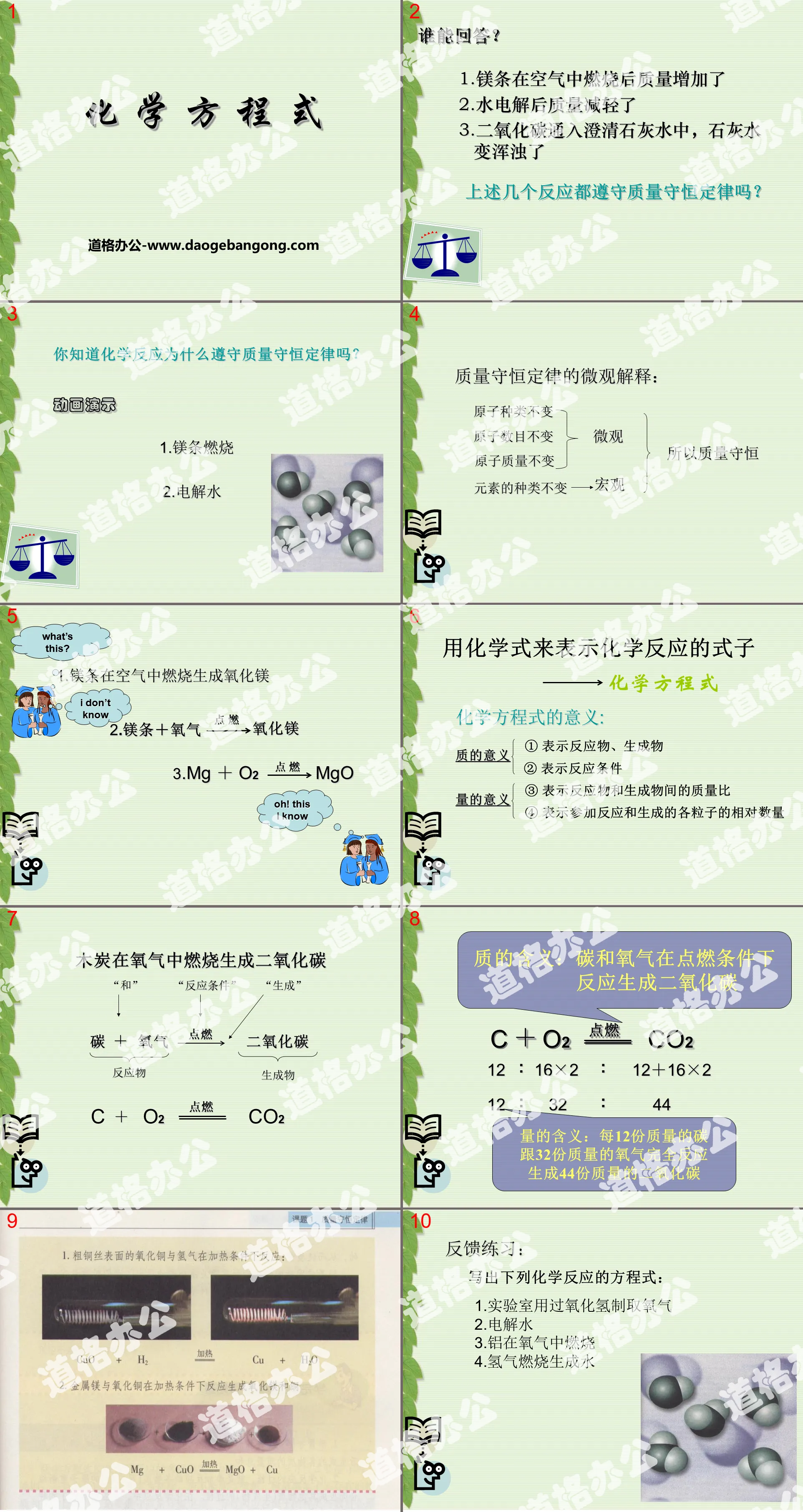
Combination reaction: A reaction in which two or more substances produce one substance.
Iron + oxygen → ferric oxide
Oxidation reaction: The reaction between a substance and oxygen.
Slow oxidation: Oxidation that proceeds very slowly and is not easily noticeable is called slow oxidation.
Determine whether the following reaction is a combination reaction or an oxidation reaction
Sulfur + oxygen → sulfur dioxide
Zinc + hydrochloric acid → zinc chloride + hydrogen
Paraffin + oxygen → carbon dioxide + water
Magnesium + oxygen → magnesium oxide
【summary】
chemical properties of oxygen
Oxygen is a gas with relatively active chemical properties. It has oxidizing properties, can support combustion, and can react with many substances. The higher the oxygen content, the more intense the combustion.
Keywords: air and water teaching courseware, oxygen teaching courseware, Hunan Education Edition ninth grade chemistry PPT courseware download, ninth grade chemistry slide courseware download, air and water PPT courseware download, oxygen PPT courseware download, .PPT format;
For more information about the "Air and Water Oxygen" PPT courseware, please click the Air and Water ppt Oxygen ppt tag.
"Air and Oxygen" PPT download:
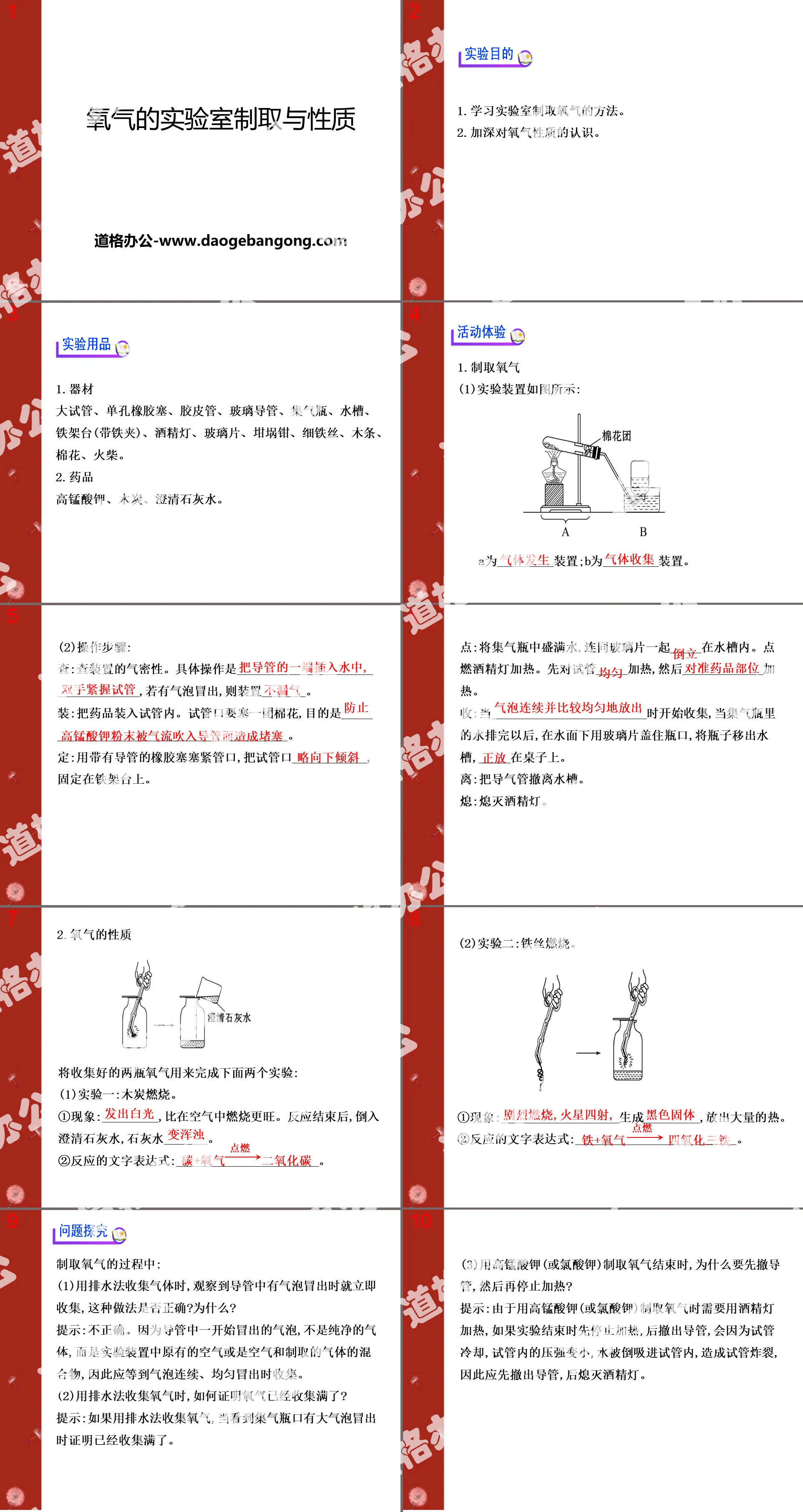
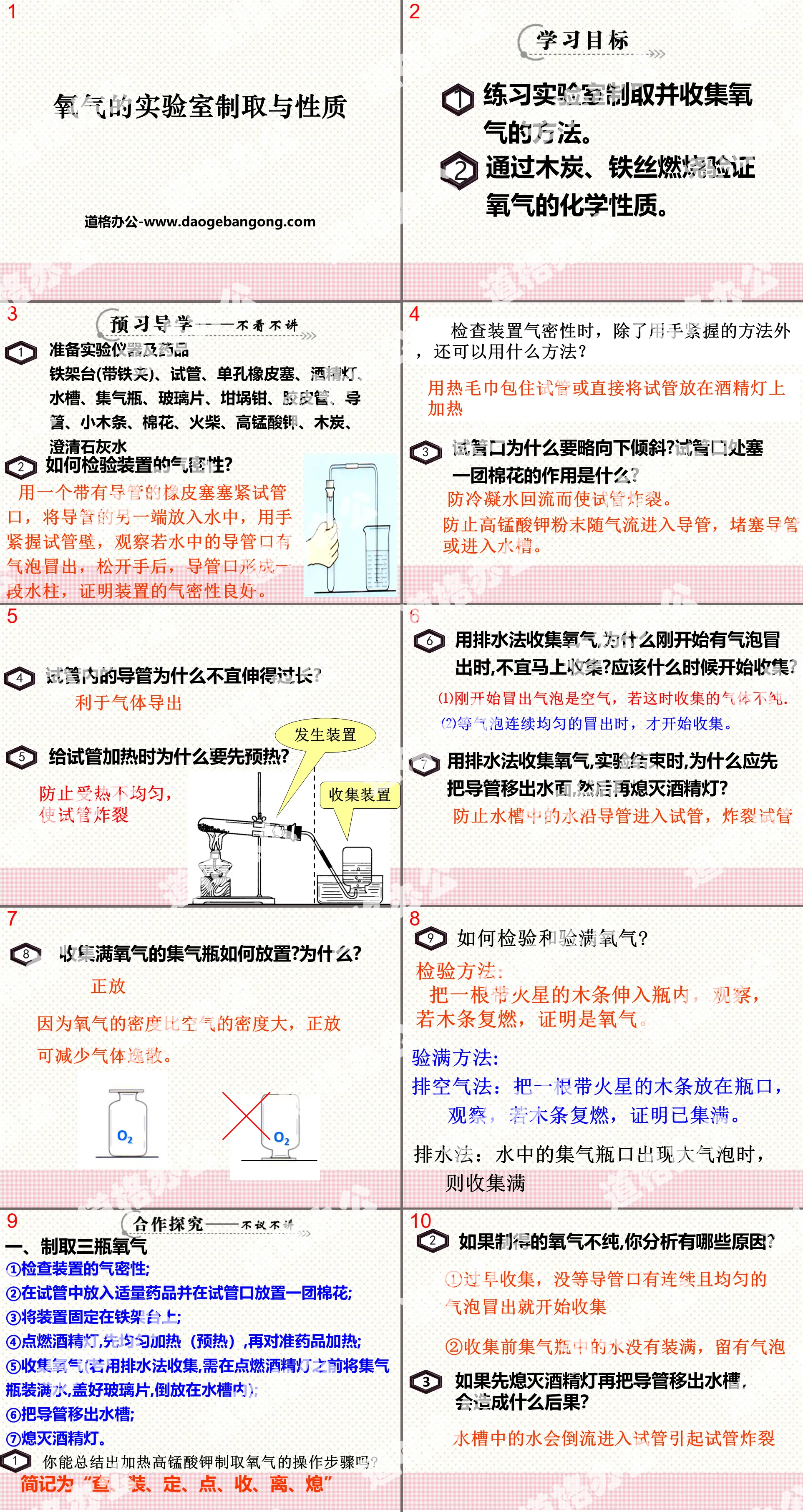
"Air and Oxygen" PPT Download Part One: Preparing Oxygen in the Laboratory 1. Steps to produce oxygen using hydrogen peroxide (hydrogen peroxide): 1. Check the air tightness of the device; 2. Add MnO2 to the flask; 3. Connect the device; 4. Add H2O2 to the separatory funnel; 5..
"Air and Oxygen" PPT courseware:
"Air and Oxygen" PPT courseware Part 1: Composition of air 1. Prove that the air contains carbon dioxide: -----Air can make clear lime water turbid. 2. Proof that there is water vapor in the air: -----When ice is placed in a cup, water droplets appear on the wall of the cup. -----Anhydrous sulfur..
"Air and Oxygen" PPT:
"Air and Oxygen" PPT Part One: Properties of Oxygen 1. Observe the color and state of a bottle of oxygen. 2. Gently fan the mouth of the bottle with your hand to let a small amount of oxygen float into your nostrils and smell its smell. Oxygen is a ____color____odorous gas. Smell...