People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Hunan Education Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"Multicomponent Air" Air and Water PPT Courseware
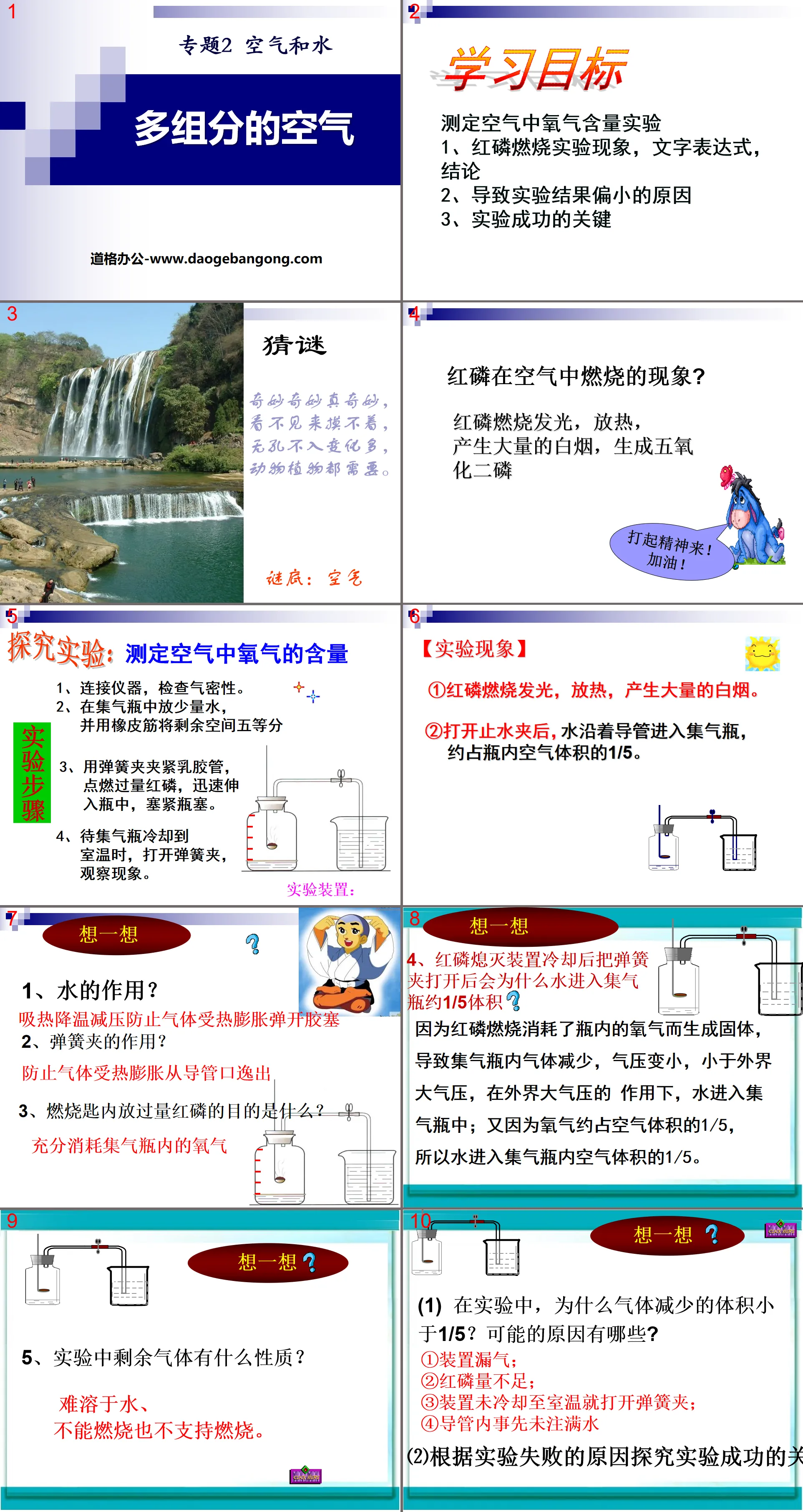
learning target
Experiment to measure the oxygen content in the air
1. Red phosphorus combustion experimental phenomena, text expressions, and conclusions
2. Reasons for the small experimental results
3. The key to successful experiment
What happens when red phosphorus burns in the air?
Red phosphorus burns, emits heat, produces a large amount of white smoke, and generates phosphorus pentoxide.
Research experiment: Determining the amount of oxygen in the air
1. Connect the instrument and check the air tightness.
2. Put a small amount of water in the gas collecting bottle and use rubber bands to divide the remaining space into five equal parts.
3. Clamp the latex tube with a spring clamp, ignite excess red phosphorus, quickly insert it into the bottle, and tighten the cork.
4. When the gas collecting bottle cools to room temperature, open the spring clamp and observe the phenomenon.
【Experimental phenomena】
① Red phosphorus burns, emits heat, and produces a large amount of white smoke.
② After opening the water stop clamp, water enters the air collecting bottle along the conduit, accounting for about 1/5 of the air volume in the bottle.
think about it
1. What is the role of water?
Absorb heat, cool down, and reduce pressure to prevent the gas from expanding due to heat and popping the rubber plug.
2. What is the function of spring clip?
Prevent gas from escaping from the pipe opening due to thermal expansion
3. What is the purpose of releasing excess red phosphorus in the combustion spoon?
Fully consume the oxygen in the gas cylinder
4. After the red phosphorus extinguishing device is cooled and the spring clip is opened, why will water enter about 1/5 of the volume of the gas collecting bottle?
Because the red phosphorus burns and consumes the oxygen in the bottle to form a solid, the gas in the gas collecting bottle decreases and the air pressure becomes smaller, which is less than the external atmospheric pressure. Under the action of the external atmospheric pressure, water enters the gas collecting bottle; and because oxygen accounts for about 10% of the air 1/5 of the volume, so the water enters 1/5 of the air volume in the gas collecting bottle.
High School Entrance Examination Link
The device shown in the picture below can be used to measure the oxygen content in the air. Before the experiment, add a small amount of water to the gas collecting bottle and mark it. Which of the following statements is incorrect ( )
A. There must be an excess of red phosphorus during the experiment
B. Use spring clamps to clamp the latex tube before igniting the red phosphorus.
C. Open the spring clip immediately after the red phosphorus goes out
D. The final volume of water entering the bottle is approximately the volume of oxygen
think
1. What would our world look like if there was no air in the world we live in?
2. The air is all around you. Can you describe its physical properties?
3. Is air a single substance? What are its main components?
Class exercises
1. Calculated by volume, the most abundant gas in the air is ( )
A oxygen B nitrogen
C carbon dioxide D rare gas
2. The volume of oxygen in 100 liters of air is approximately ( )
A 78 liters B 78% C 21 liters D 21%
3. The volume ratio of oxygen to nitrogen in the air is approximately ( )
A 4:1 B 1:4 C 1:5 D 5:1
4. Pure substances and mixtures
Pure substance: composed of a substance
Such as: N2, O2, CO2, P, P2O5, H2O
Mixture: Made up of several substances
Such as: air, sea water, river water, mineral water, etc.
Pure substances can be represented by special chemical symbols. For example, nitrogen, oxygen, and carbon dioxide can be represented as N2, O2, CO2, etc. respectively.
Class exercises
1. Which of the following substances is pure ( )
A A rare gas separated from the air
Part B frozen distilled water
C salt water
D 75% medicinal alcohol
2. Among the following groups of substances, the former is a single substance and the latter is a mixture ( )
A. Nitrogen Distilled water B. iron ore salt water
C. Hydrogen CaCO3 D. carbon dioxide air
3. Which of the following water is a pure substance ( )
A mineral water B tap water C river water D ice-floating water
3. Air pollution and its prevention
1. Harmful substances in the air
Harmful gases: sulfur dioxide (SO2), carbon monoxide (CO), nitrogen dioxide (N02)
Smoke (respirable particulate matter)
2. Three major types of pollution
SO2, NO2, etc. → acid rain
Freon, etc.→Ozone Hole
Increased CO2 content →greenhouse effect
Keywords: air and water teaching courseware, multi-component air teaching courseware, Hunan Education Edition ninth-grade chemistry PPT courseware download, ninth-grade chemistry slide courseware download, air and water PPT courseware download, multi-component air PPT courseware Download, .PPT format;
For more information about the "Air and Water Multi-Component Air" PPT courseware, please click the Air and Water ppt Multi-Component Air ppt tag.
"Multicomponent Air" Air and Water PPT Courseware 3:
"Multicomponent Air" Air and Water PPT Courseware 3 1. Composition of Air [Think about it]: What is air made of? In order to understand the composition of air, many scientists have devoted themselves to research in this area, such as Swedish chemist Scheele and British chemist Priest.
"Multicomponent Air" Air and Water PPT Courseware 2:
"Multicomponent Air" Air and Water PPT Courseware 2 Thoughts 1. If there was no air in the world we live in, what would our world look like? 2. The air is all around you. Can you describe its physical properties? 3. Air is a single...
File Info
Update Time: 2024-11-24
This template belongs to Chemistry courseware Hunan Education Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Multicomponent Air" Air and Water PPT Courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Multicomponent Air" Air and Water PPT Courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Multicomponent Air" Air and Water PPT Courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview