"Laboratory Production Method of Carbon Dioxide" The World of Carbon PPT Courseware 2 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Laboratory Production M... | 2875次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Laboratory Production Method of Carbon Dioxide" The World of Carbon PPT Courseware 2 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Laboratory Production Method of Carbon Dioxide" The World of Carbon PPT Courseware 2, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)

Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see







Authoritative PPT Summary
"Laboratory Production Method of Carbon Dioxide" The World of Carbon PPT Courseware 2
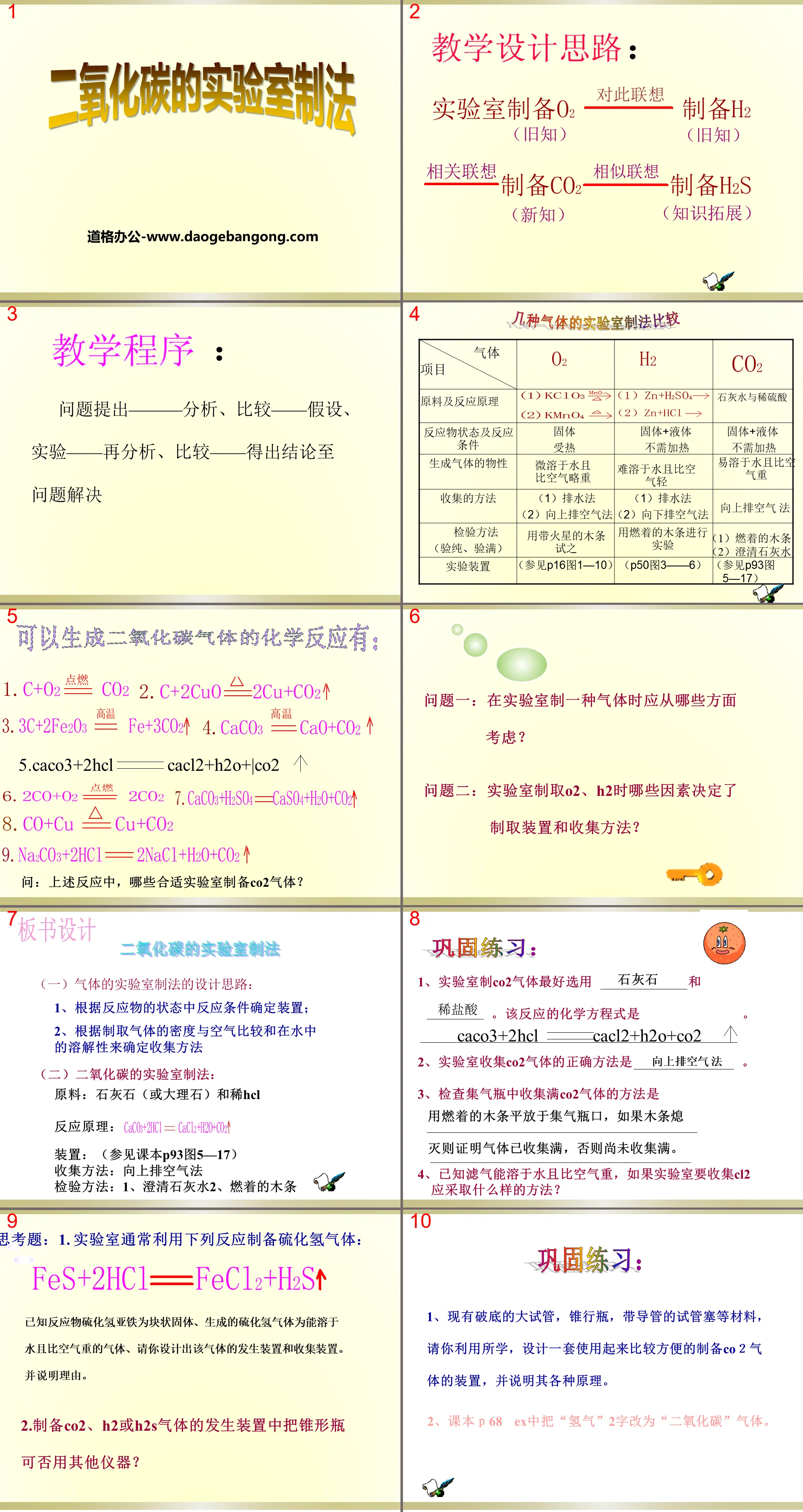
Teaching program
Problem raising - analysis, comparison - hypothesis, experiment - re-analysis, comparison - drawing conclusions to problem solving
Question 1: What aspects should be considered when preparing a gas in the laboratory?
Question 2: What factors determine the preparation device and collection method when preparing O2 and H2 in the laboratory?
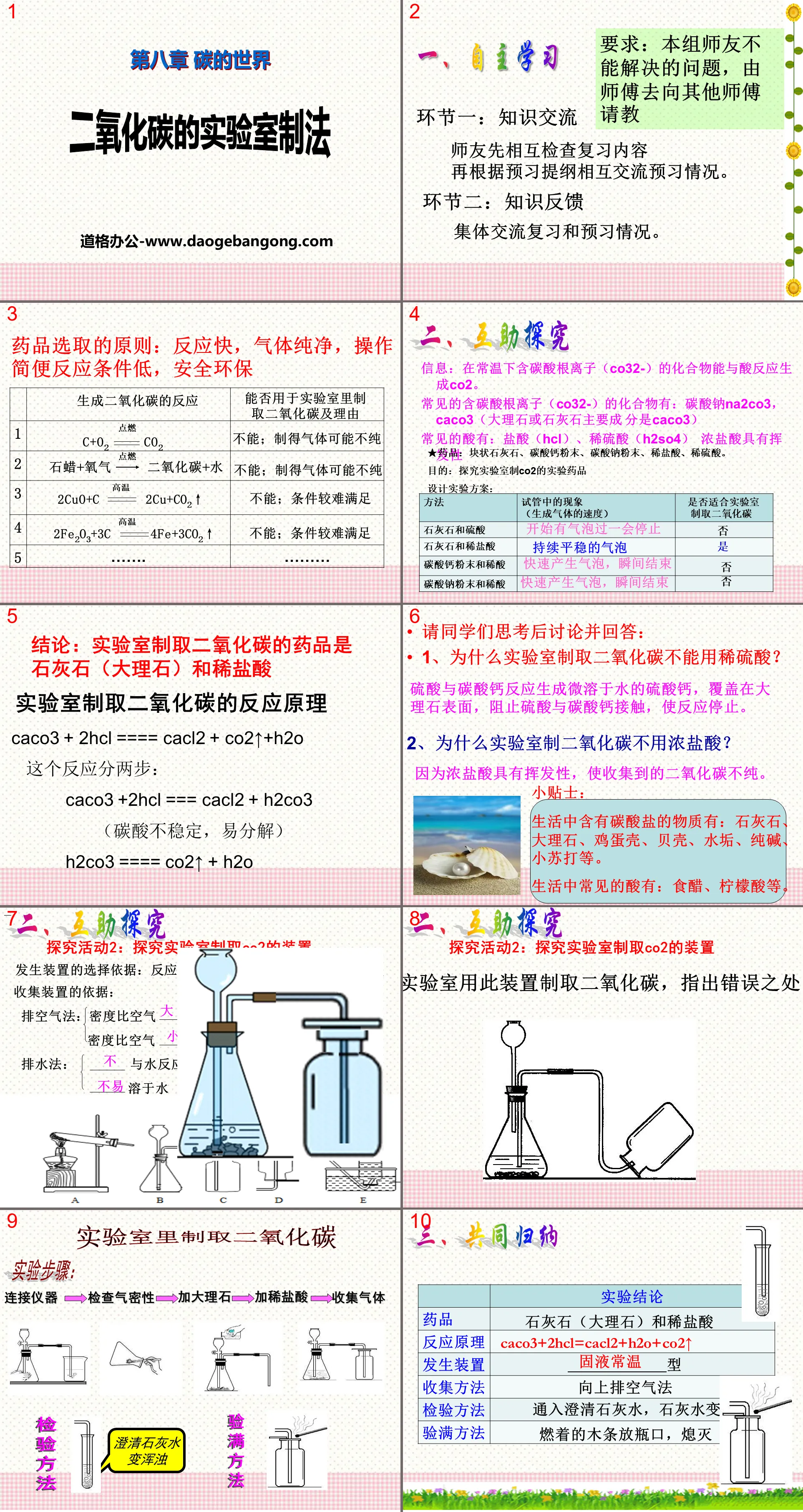
Laboratory preparation of carbon dioxide
(1) Design ideas for laboratory gas preparation methods:
1. Determine the device according to the reaction conditions in the state of the reactants;
2. Determine the collection method based on the density of the produced gas compared with air and the solubility in water.
(2) Laboratory preparation method of carbon dioxide:
Raw materials: limestone (or marble) and dilute HCl
Reaction principle:
Device: (See Figure 5-17 on P93 of the textbook)
Collection method: upward exhaust air method
Inspection methods: 1. Clarified lime water 2. Burning wood sticks
Consolidation exercises:
1. It is best to use limestone and dilute hydrochloric acid to produce CO2 gas in the laboratory. The chemical equation of this reaction is CaCO3+2HCl==CaCl2+H2O+CO2.
2. The correct way to collect CO2 gas in the laboratory is to exhaust the air upward.
3. The way to check whether the CO2 gas is fully collected in the gas collecting bottle is to place a burning wooden stick flatly on the mouth of the gas collecting bottle. If the wooden stick goes out, it means that the gas has been fully collected, otherwise it has not been fully collected.
4. It is known that filter gas can be dissolved in water and is heavier than air. If the laboratory wants to collect Cl2, what method should be used?
Questions to think about: 1. The laboratory usually uses the following reactions to prepare hydrogen sulfide gas:
It is known that the reactant ferrous hydrogen sulfide is a massive solid, and the generated hydrogen sulfide gas is a gas that is soluble in water and heavier than air. Please design a generating device and a collection device for this gas. and explain the reasons.
2. Can other instruments be used for the Erlenmeyer flask in the generating device for preparing CO2, H2 or H2S gas?
Consolidation exercises:
1. There are existing large test tubes with broken bottoms, Erlenmeyer flasks, test tube plugs with conduits and other materials. Please use what you have learned to design a device for preparing CO2 gas that is more convenient to use, and explain its various principles.
2. In the textbook P68 Ex, the word "hydrogen" is changed to "carbon dioxide" gas.
Keywords: The world of carbon teaching courseware, the laboratory production method of carbon dioxide teaching courseware, the Beijing curriculum reform version of the ninth grade chemistry PPT courseware download, the ninth grade chemistry slide courseware download, the world of carbon PPT courseware download, the laboratory production of carbon dioxide Download French PPT courseware in .PPT format;
For more information about the PPT courseware "Laboratory Method for Preparing Carbon Dioxide in the World of Carbon", please click the "Laboratory Method for Preparing Carbon Dioxide in the World of Carbon" ppt tag.
"Laboratory Production Method of Carbon Dioxide" The World of Carbon PPT courseware:
"Laboratory Method for Preparing Carbon Dioxide" The World of Carbon PPT courseware 1. Independent learning requirements: For problems that the teachers in this group cannot solve, the master will ask other teachers for advice. Session 1: Knowledge exchange. The teachers and friends will first check each other's review content and then follow the preview outline. Communicate with each other...