People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| People's Education Press Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"Laboratory Preparation and Properties of Oxygen" Air Around Us PPT Courseware 2
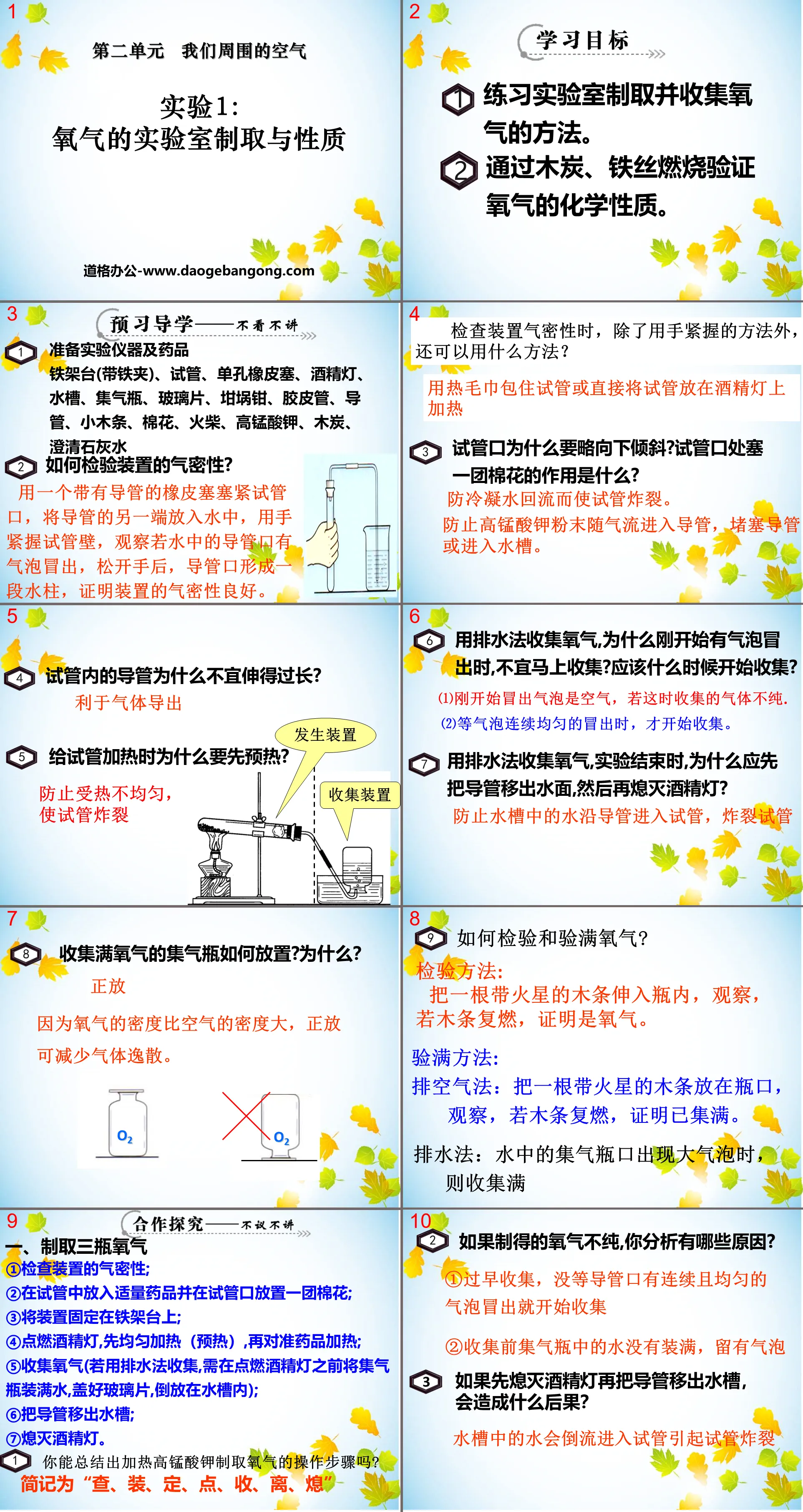
learning target
1. Practice the method of preparing and collecting oxygen in the laboratory.
2. Verify the chemical properties of oxygen by burning charcoal and iron wire.
Pre-study tutorial
1. Prepare experimental equipment and drugs
Iron stand (with iron clamp), test tube, single-hole rubber stopper, alcohol lamp, sink, gas bottle, glass piece, crucible tongs, rubber tube, conduit, small wooden strips, cotton, matches, potassium permanganate, charcoal, clarification Lime water
2. How to check the air tightness of the device?
Plug the mouth of the test tube tightly with a rubber stopper with a catheter, put the other end of the catheter into the water, hold the wall of the test tube tightly with your hand, and observe if bubbles emerge from the mouth of the catheter in the water. After releasing your hand, the mouth of the test tube forms a section. The water column proves that the air tightness of the device is good.
When checking the air tightness of the device, besides holding it tightly with your hands, what other methods can be used?
Wrap the test tube with a hot towel or place the test tube directly on an alcohol lamp to heat it
3. Why should the mouth of the test tube be tilted slightly downward? What is the purpose of stuffing a ball of cotton at the mouth of the test tube?
Prevent the condensed water from flowing back and causing the test tube to explode.
Prevent potassium permanganate powder from entering the duct with the air flow, blocking the duct or entering the sink.
collaborative inquiry
1. Prepare three bottles of oxygen
① Check the air tightness of the device;
②Put an appropriate amount of medicine into the test tube and place a ball of cotton at the mouth of the test tube;
③Fix the device on the iron stand;
④Light the alcohol lamp, heat it evenly (preheat) first, and then heat it towards the medicine;
⑤ Collect oxygen (if you use the drainage method to collect it, you need to fill the gas collecting bottle with water before lighting the alcohol lamp, cover it with a glass piece, and place it upside down in the sink);
⑥Move the pipe out of the sink;
⑦ Extinguish the alcohol lamp.
1. Can you summarize the steps for heating potassium permanganate to produce oxygen?
The abbreviation is "check, install, fix, point, close, leave, and extinguish"
2. If the oxygen produced is impure, what are the reasons?
① Premature collection, starting collection before continuous and even bubbles emerge from the catheter port
②The water in the gas collecting bottle is not full before collection, leaving bubbles
3. What will be the consequences if the alcohol lamp is extinguished first and then the catheter is removed from the sink?
The water in the sink will flow back into the test tube and cause the test tube to burst.
2. Verify the properties of oxygen
1. Reaction of charcoal and oxygen
(1) When inserting red-hot charcoal into oxygen, why should it be inserted slowly from the mouth of the bottle downwards?
Carbon dioxide is denser than air and does not burn or support combustion. The carbon dioxide produced by burning charcoal accumulates at the bottom of the bottle and will extinguish the charcoal.
(2) What are the phenomena of charcoal burning? After the combustion stops, add a small amount of clarified lime water to the gas collecting bottle and shake it. What phenomena can be observed?
①Charcoal produces white light ②Clear lime water turns turbid
(3) Write a literal expression for the reaction between charcoal and oxygen.
2. Oxygen reacts with iron wire
(1) Why should the match be inserted into the oxygen bottle when it is almost burned out?
(2) What is the purpose of pre-filling a small amount of water or sand in the bottle?
(3) Accurately describe the experimental phenomenon and write a text expression of the reaction.
Keywords: The air around us teaching courseware, the laboratory preparation and properties of oxygen teaching courseware, the People's Education Press version of the ninth grade chemistry PPT courseware download, the ninth grade chemistry slide courseware download, the air around us PPT courseware download, oxygen Laboratory preparation and properties PPT courseware download, .PPT format;
For more information about "Laboratory Preparation and Properties of Oxygen in the Air Around Us" PPT courseware, please click on the "Laboratory Preparation and Properties of Oxygen in the Air Around Us" ppt tag.
"Laboratory Preparation and Properties of Oxygen" PPT Courseware 4:
"Laboratory Preparation and Properties of Oxygen" PPT Courseware 4 Experimental Purpose 1. Learn the method of producing oxygen in the laboratory. 2. Deepen your understanding of the properties of oxygen. Experimental supplies 1. Equipment: large test tube, single-hole rubber stopper, rubber tube, glass tube, gas bottle, sink, iron frame...
"Laboratory Preparation and Properties of Oxygen" PPT Courseware 3:
"Laboratory Preparation and Properties of Oxygen" PPT courseware 3 1. Producing oxygen by heating potassium permanganate 1. Experiment purpose: Preparing oxygen and exploring the properties of oxygen 2. Experimental principle: 3. Instruments and medicines: iron stand, Iron clamps, test tubes, single-hole rubber stoppers, air tubes, collection...
"Laboratory Preparation and Properties of Oxygen" PPT Courseware 2:
"Laboratory Preparation and Properties of Oxygen" PPT Courseware 2 Learning Objectives 1. Practice the method of producing and collecting oxygen in the laboratory. 2. Verify the chemical properties of oxygen by burning charcoal and iron wire. Pre-study tutorial 1. Prepare experimental instruments and drugs, iron stand (with iron clamps), test tubes...
File Info
Update Time: 2024-11-10
This template belongs to Chemistry courseware People's Education Press Ninth Grade Chemistry Volume 1 industry PPT template
"Laboratory Preparation and Properties of Oxygen" Air Around Us PPT Courseware 2 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Laboratory Preparation and Properties of Oxygen" Air Around Us PPT Courseware 2 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Laboratory Preparation and Properties of Oxygen" Air Around Us PPT Courseware 2, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview