People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Hunan Education Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
People's Education Press High School Chemistry Compulsory Course 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Hunan Education Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"Laboratory Preparation and Properties of Carbon Dioxide" PPT courseware
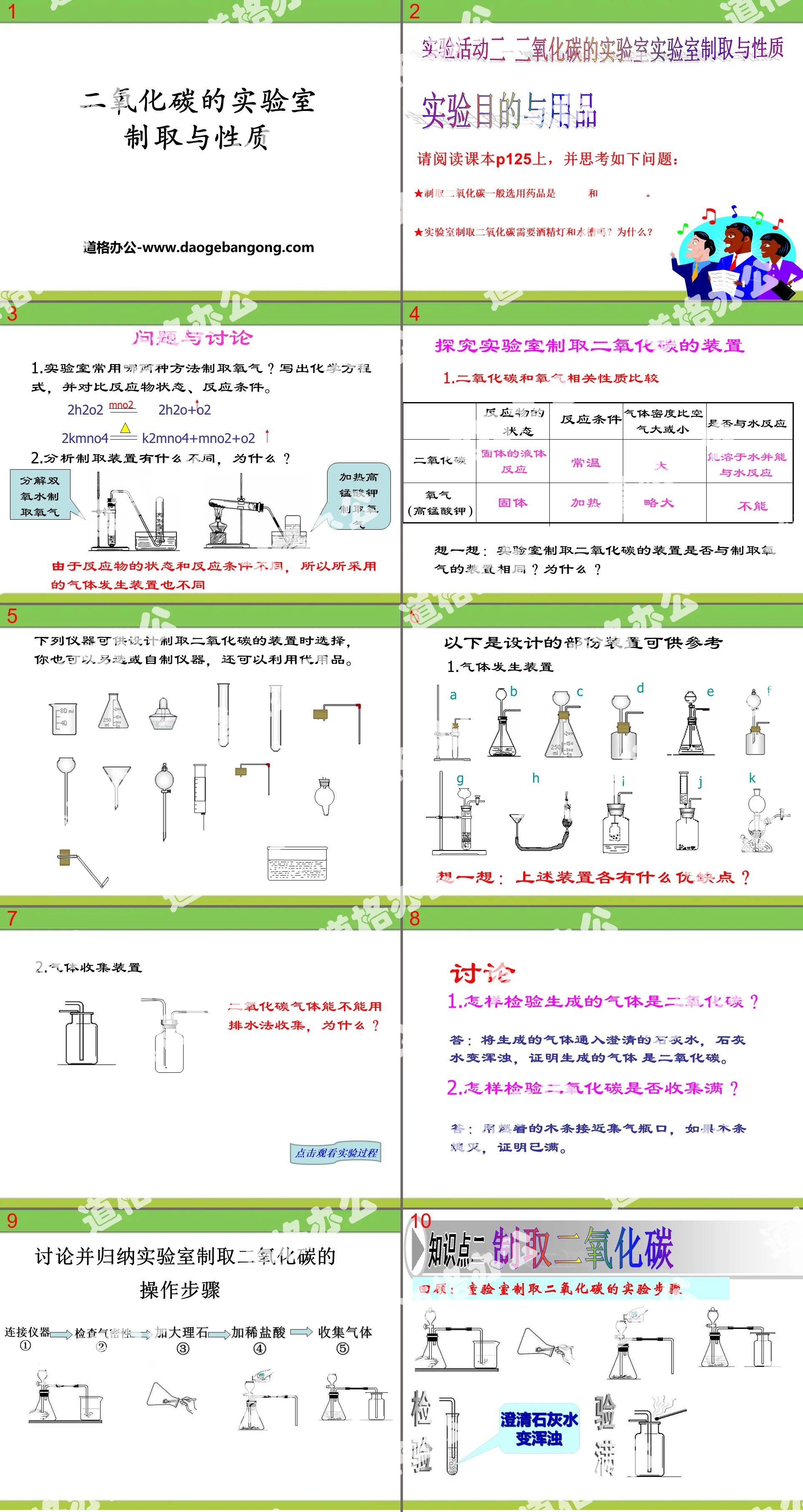
Experiment purpose and supplies
Please read textbook P125 and think about the following questions:
The commonly used drugs for producing carbon dioxide are _________ and _______________.
Does producing carbon dioxide in the laboratory require an alcohol lamp and a water tank? Why?
Questions and Discussion
1. What two methods are commonly used in laboratories to produce oxygen? Write chemical equations and compare the states of reactants and reaction conditions.
2H2O2==2H2O+O2
2KMnO4==K2MnO4+MnO2+O2
2. What are the differences between analysis and preparation devices and why?
Since the state of the reactants and reaction conditions are different, the gas generating devices used are also different.
discuss
1. How to check whether the generated gas is carbon dioxide?
Answer: Pass the generated gas into clear lime water. If the lime water becomes turbid, it proves that the generated gas is carbon dioxide.
2. How to check whether the carbon dioxide is fully collected?
Answer: Use a burning wooden stick close to the mouth of the gas collecting bottle. If the wooden stick goes out, it means it is full.
Exploring the properties of carbon dioxide
(1) Drop litmus into white vinegar and the solution will turn ____ color
(2) Drop the litmus solution into water to observe the phenomenon; pass carbon dioxide into a test tube containing dry litmus paper and observe the phenomenon.
(3) Pour carbon dioxide into the purple litmus solution and observe the phenomenon.
(4) Heat the discolored solution slightly and observe the color change.
Carbon dioxide reacts with water
Phenomenon: Put the florets sprayed with water into carbon dioxide, and the purple litmus test solution turns red.
CO2 + H2O = H2CO3
Phenomenon: When heated, carbonic acid decomposes and carbon dioxide escapes from the solution, so the red litmus test solution turns purple again.
Carbonic acid is very unstable and breaks down easily
H2CO3=H2O+CO2↑
Why is there a film on the wall of the reagent bottle that has been holding lime water for a long time? How might it have come about?
The component of lime water is an aqueous solution of calcium hydroxide. Carbon dioxide in the air will react with calcium hydroxide to produce calcium carbonate precipitation. Because carbon dioxide comes into contact with the surface of the solution, a white film is formed on the surface of the solution. The reaction that occurs is:
CO2 + Ca(OH)2 = CaCO3 ↓+ H2O
calcium carbonate
What problems did you find during the experiment? How did you solve it? Students communicate.
1. How to prove experimentally that carbonated drinks (such as soda) contain carbon dioxide?
2. Can other properties experiments be designed?
3. After-school homework and family experiments:
Use household kitchen supplies to produce carbon dioxide gas.
Keywords: Laboratory Preparation and Properties of Carbon Dioxide Teaching Courseware, Hunan Education Edition Ninth Grade Chemistry PPT Courseware Download, Ninth Grade Chemistry Slide Courseware Download, Laboratory Preparation and Properties of Carbon Dioxide PPT Courseware Download, .PPT format;
For more information about the "Laboratory Preparation and Properties of Carbon Dioxide" PPT courseware, please click on the "Laboratory Preparation and Properties of Carbon Dioxide" ppt tab.
"Laboratory Preparation and Properties of Carbon Dioxide" PPT Courseware 2:
"Laboratory Preparation and Properties of Carbon Dioxide" PPT courseware 2 1. The status and role of teaching materials This lesson mainly studies how to prepare carbon dioxide in the laboratory and verify the properties of carbon dioxide. It occupies a very important position in the entire teaching material system. Also, learn well...
"Laboratory Preparation and Properties of Carbon Dioxide" Carbon and Carbon Oxides PPT Courseware 7:
"Laboratory Preparation and Properties of Carbon Dioxide" Carbon and Carbon Oxides PPT Courseware 7 Experimental Objectives: 1. Practice the method of producing carbon dioxide in the laboratory and collecting the gas using the upward air exhaust method. 2. Do experiments on the properties of carbon dioxide to deepen your understanding of the properties of carbon dioxide..
"Laboratory Preparation and Properties of Carbon Dioxide" Carbon and Carbon Oxides PPT Courseware 6:
"Laboratory Preparation and Properties of Carbon Dioxide" Carbon and Carbon Oxides PPT Courseware 6 Learning Objectives: 1. Knowledge and Abilities: Learn the methods of producing carbon dioxide in the laboratory and know the properties and uses of carbon dioxide 2. Processes and methods: Through carbon dioxide Preparation of learning..
File Info
Update Time: 2024-10-21
This template belongs to Chemistry courseware Hunan Education Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Laboratory Preparation and Properties of Carbon Dioxide" PPT courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Laboratory Preparation and Properties of Carbon Dioxide" PPT courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Laboratory Preparation and Properties of Carbon Dioxide" PPT courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview