People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| People's Education Press High School Chemistry Compulsory Course I | pptx | 6 MB |
Description
"Laboratory Preparation Method of Chlorine and Testing of Cl-" Chlorine and its Compounds PPT
Part One: Goals and Competencies:
1. Understand the principles and experimental equipment for producing Cl2 in the laboratory. (Scientific inquiry and innovation consciousness)
2. Master the detection method of Cl-. (Macroscopic identification and microscopic analysis)
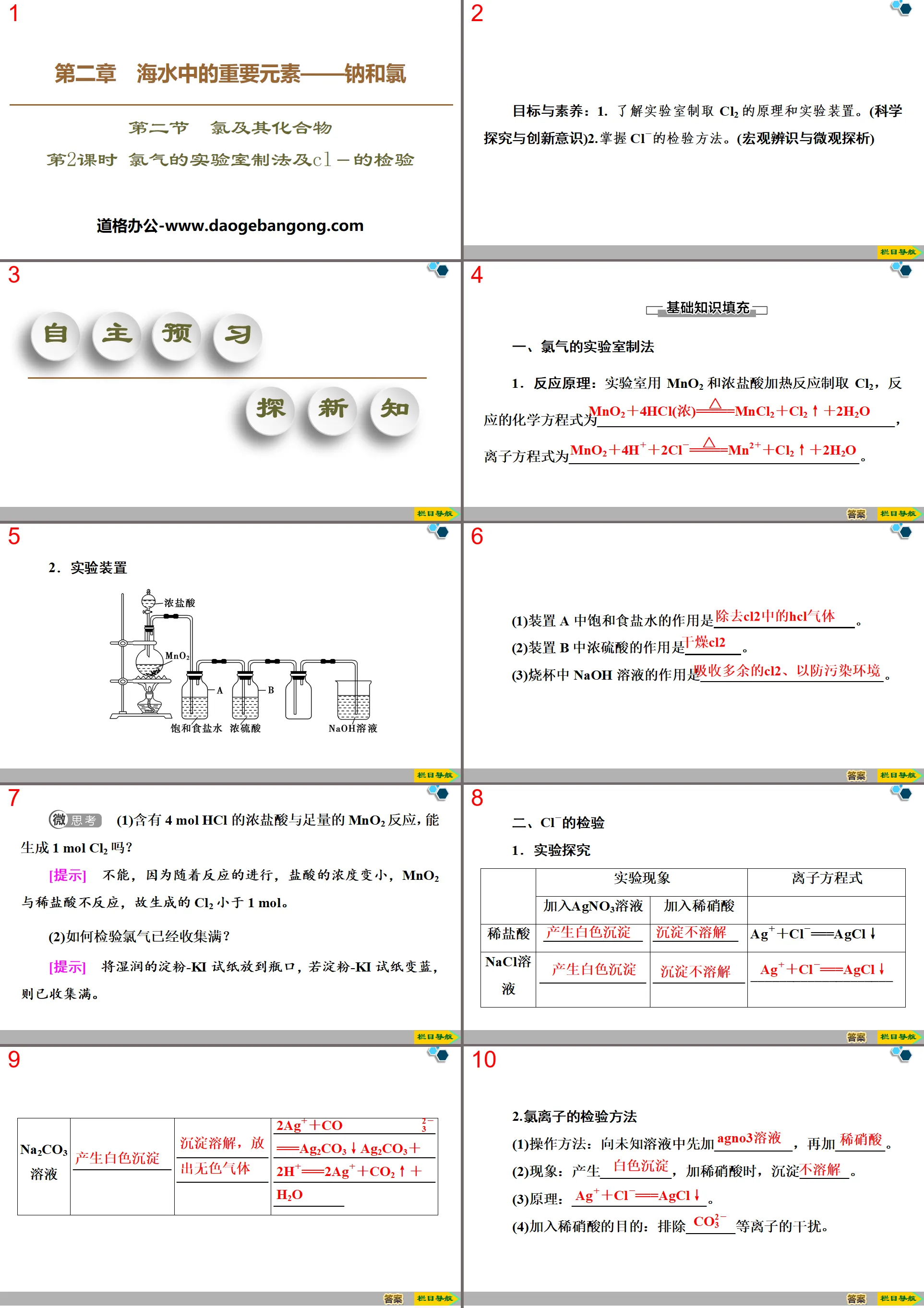
Laboratory preparation method of chlorine and test of Cl- PPT, part 2: independent preview to explore new knowledge
1. Laboratory preparation method of chlorine gas
1. Reaction principle: Cl2 is produced by heating MnO2 and concentrated hydrochloric acid in the laboratory. The chemical equation of the reaction is ____________________________________________,
The ionic equation is_________________________________________.
2. experimental device
(1) The function of saturated salt water in device A is _______________.
(2) The function of concentrated sulfuric acid in device B is ____________.
(3) The function of the NaOH solution in the beaker is _______________.
(1) Can concentrated hydrochloric acid containing 4 mol HCl react with a sufficient amount of MnO2 to produce 1 mol Cl2?
[Tips] No, because as the reaction proceeds, the concentration of hydrochloric acid becomes smaller, and MnO2 does not react with dilute hydrochloric acid, so the Cl2 generated is less than 1 mol.
(2) How to check that the chlorine gas has been fully collected?
[Tips] Place the moist starch KI test paper into the mouth of the bottle. If the starch KI test paper turns blue, the collection is full.
2. Test of Cl-
1. Experimental exploration
2. Testing methods for chloride ions
(1) Operation method: Add ___________ to the unknown solution first, then add _______.
(2) Phenomenon: __________ is produced, and when dilute nitric acid is added, _______ precipitates.
(3)Principle: ____________________.
(4) The purpose of adding dilute nitric acid is to eliminate interference from _______ plasma.
The laboratory preparation method of chlorine and the testing of Cl- PPT, the third part: core breakthroughs and difficult difficulties
Gas preparation unit analysis
1. Common gas generating devices
2. Common purification devices - used to remove impurity gases from gases
3. Common gas collection devices
4. Common gas measuring device - measuring the volume of gas through liquid drainage method
5. Toxic gas treatment device
1. A chemical interest group uses MnO2 and concentrated hydrochloric acid to prepare Cl2 with the device as shown. Which of the following analysis is incorrect ()
A. ①A separatory funnel can be used instead of a long-neck funnel
B. ① lack of heating device
C. ②The NaOH solution contained in the container can purify chlorine gas
D. ④The NaOH solution contained in the container can absorb exhaust gas
C. In A, a separatory funnel can be used instead of a long-neck funnel to avoid volatilization of hydrochloric acid and gas escape, and to facilitate control of the reaction rate. A is correct; manganese dioxide and concentrated hydrochloric acid need to be heated to react to generate chlorine, and a heating device needs to be added. , B is correct; the NaOH solution contained in B absorbs chlorine and cannot purify Cl2. You can use saturated salt water to purify the chlorine. C is incorrect; the sodium hydroxide solution reacts with chlorine and can absorb chlorine to prevent air pollution. D is correct. ]
Laboratory preparation method of chlorine and Cl- test PPT, part 4: meeting standards in class and improving literacy
1. A chemistry group uses the device shown in the figure to produce chlorine gas. Which of the following statements is incorrect ()
A. There are at least three obvious errors in the device diagram
B. The method used to collect chlorine gas in this experiment is incorrect.
C. In order to prevent chlorine from polluting the air, exhaust gas treatment must be carried out
D. Put a piece of moist starch potassium iodide test paper at the conduit opening of the gas collecting bottle to confirm whether there is Cl2 escaping.
B: The device was not heated with an alcohol lamp. A separatory funnel should have been used. There was no exhaust gas absorption device. There were at least three obvious errors. ]
2. Anhydrous ferric chloride can be prepared using the following apparatus. Which of the following statements is correct ()
A. The reactants for preparing chlorine in flask B are manganese dioxide and dilute hydrochloric acid
B. Devices C and D contain concentrated sulfuric acid and saturated salt water respectively.
C. A drying device needs to be added between device E and device F to produce anhydrous ferric chloride.
D. The purpose of device F is to check whether chlorine gas escapes
C. The drying device should be behind the impurity removal device. Devices C and D contain saturated salt water and concentrated sulfuric acid respectively. The water vapor evaporated in device F can easily enter device E retrogradely, so it needs to be between devices E and F. Add a drying device; Device F is an exhaust gas absorption device. ]
3. Chlorine is commonly used in water plants to sterilize domestic water. In order to make huge profits, some unscrupulous traders in the market use such tap water to pass off as pure water (the concentration of ions is very low) and sell it, which has caused certain adverse effects on people's lives. Among the following chemical reagents, the one that can be used to identify this kind of tap water and purified water is ()
A. Phenolphthalein solution B. barium chloride solution
C. Sodium hydroxide solution D. silver nitrate solution
D. Cl2-sterilized tap water contains HCl and HClO, which can be identified with AgNO3 solution. The water that produces white precipitates is tap water, and the water that does not produce precipitates is pure water. ]
Keywords: Free download of the PPT courseware for high school chemistry compulsory course I of the People's Education Press, PPT download of laboratory preparation method of chlorine and test of Cl-, PPT download of chlorine and its compounds, .PPT format;
For more information about the PPT courseware "Chlorine and its compounds, chlorine gas laboratory preparation method and Cl- test", please click on the Chlorine and its compounds ppt Chlorine gas laboratory preparation method and Cl- test ppt tag.
"Chlorine and its Compounds" Important elements in seawater - sodium and chlorine PPT (Lesson 2 Laboratory preparation method of chlorine gas and test of chloride ions):
"Chlorine and Its Compounds" Important Elements Sodium and Chlorine in Seawater PPT (Laboratory Method for Preparing Chlorine and Testing of Chloride Ions in Lesson 2) Part One Content: Learning Objectives Course Standards 1. Be able to select experimental devices according to the reaction principle to prepare chlorine gas. 2. Preparation of chlorine gas through laboratory..
"Chlorine and its Compounds" Important elements in seawater - sodium and chlorine PPT (Lesson 1 Properties of chlorine):
"Chlorine and Its Compounds" Important Elements Sodium and Chlorine in Seawater PPT (Properties of Chlorine in Lesson 1) Part One Content: Learning Objectives Course Standards 1. Understand chlorine and its importance through application examples in real situations or through experimental exploration The main properties of compounds, identify...
"Laboratory Preparation Method of Chlorine Gas and Testing of Chloride Ions" PPT courseware on chlorine and its compounds:
"Laboratory Preparation of Chlorine and Testing of Chloride Ions" Chlorine and Its Compounds PPT Courseware Part One Content: Literacy Objectives 1. Review the experimental device of carbon dioxide learned in junior high school and combine it with the manganese dioxide and concentrated hydrochloric acid used to prepare chlorine in the laboratory. Choose appropriate experiments for your properties..
File Info
Update Time: 2024-11-22
This template belongs to Chemistry courseware People's Education Press High School Chemistry Compulsory Course I industry PPT template
"Laboratory Preparation Method of Chlorine and Testing of Cl-" Chlorine and its Compounds PPT Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Laboratory Preparation Method of Chlorine and Testing of Cl-" Chlorine and its Compounds PPT is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Laboratory Preparation Method of Chlorine and Testing of Cl-" Chlorine and its Compounds PPT, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview