"End of Chapter Review Lesson" Important elements in seawater—sodium and chlorine PPT Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "End of Chapter Review L... | 17975次 | 0.00 | Free Download |
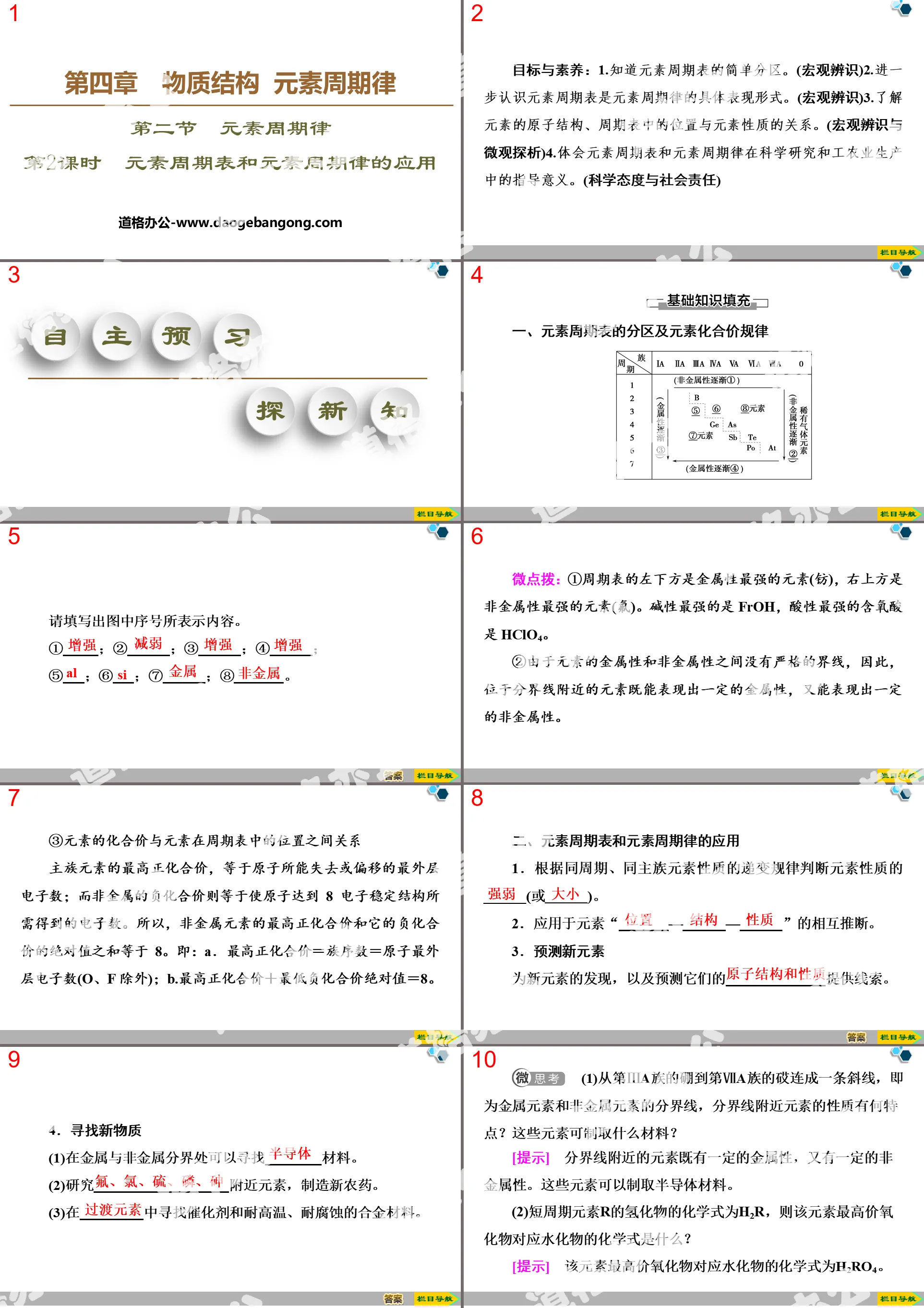
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "End of Chapter Review Lesson" Important elements in seawater—sodium and chlorine PPT is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"End of Chapter Review Lesson" Important elements in seawater—sodium and chlorine PPT, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)

Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see








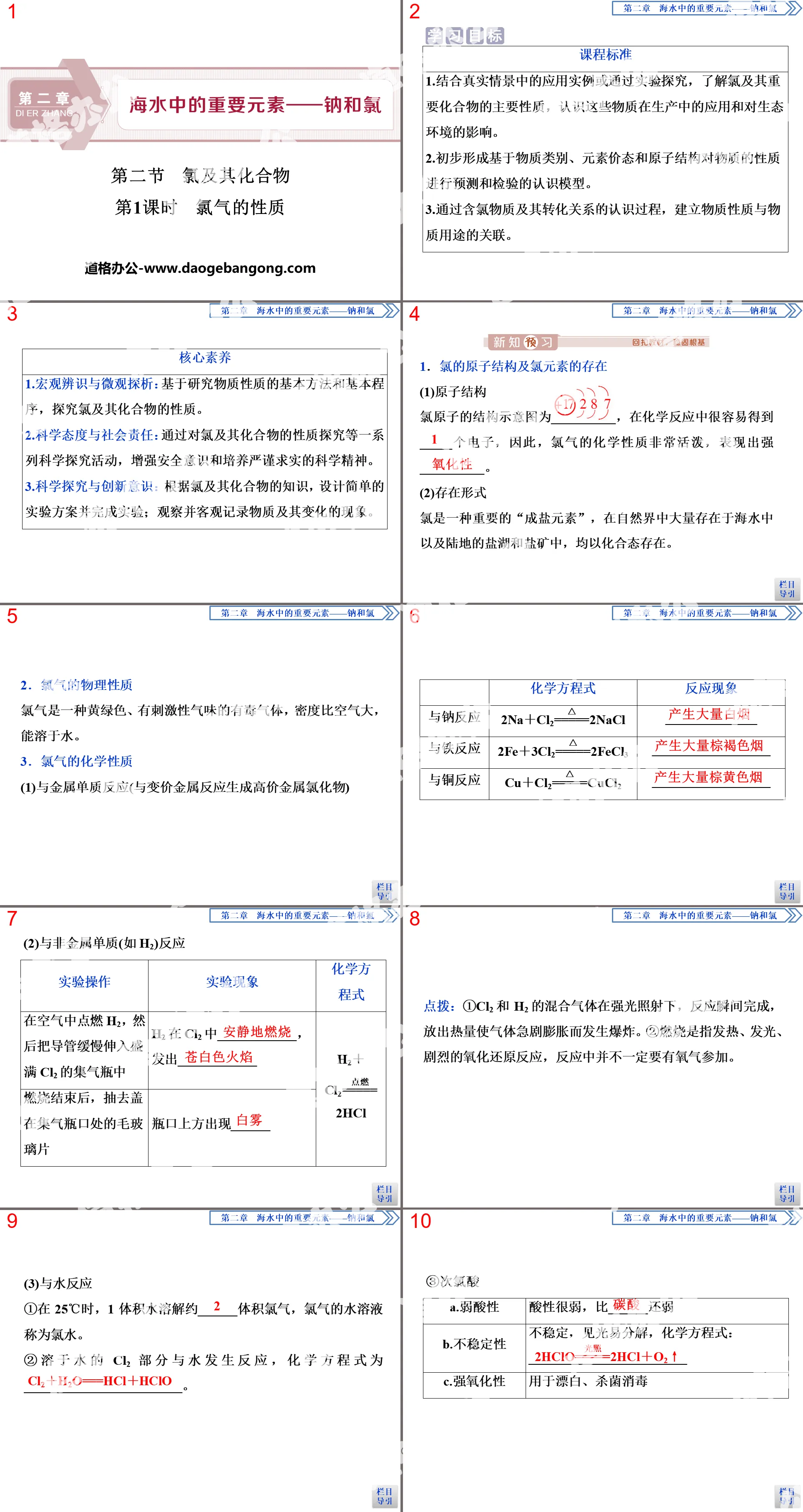
Authoritative PPT Summary
"End of Chapter Review Lesson" Important elements in seawater—sodium and chlorine PPT
Special topic summary and practice
Calculations centered on the amount of matter
1. The relationship between the amount of a substance and Avogadro's constant, gas molar volume, molar mass, and the amount concentration of the substance.
2. Calculation formula
(1)n=NNA; (2)n=mM; (3)n=VgVm; (4) cB=nBVaq.
3. Precautions
(1) Highlight the core of the quantity of matter, which is the bridge and link between other physical quantities. The general model is: calculate one physical quantity, calculate the quantity of matter, and calculate another physical quantity.
(2) Pay attention to the correspondence of units during the conversion process.
(3) When using the molar volume of gas, attention should be paid to external conditions. If it is not a standard condition, 22.4 L·mol-1 cannot be directly substituted into the calculation.
(4) Under different conditions, the state of the same substance may be different.
(5) When calculating the amount and concentration of a substance, the final destination in each case is the definition of the amount and concentration of the substance, c=n/V. Make full use of the calculation formula for the amount of the substance and use the amount of the substance as a bridge. effect.
Thematic sparring
1. Use NA to represent the value of Avogadro's constant. The following statement is correct ()
A. Under standard conditions, 11.2 L of water contains 0.5 NA water molecules.
B. In a MgCl2 solution with a substance concentration of 0.5 mol·L-1, the number of Mg2+ contained is 0.5NA
C. 1 mol of H2 and O2 contains NA molecules
D. The number of atoms contained in 11.2 L NH3 is 2NA
C: Water is not a gas under standard conditions, and the gas molar volume under standard conditions cannot be used. 22.4 L·mol-1, error A; the volume of the solution is not specified, and the number of solute particles cannot be calculated, error B; 1 mol of gas is equal Contains NA molecules, which has nothing to do with whether it is a mixed gas. C is correct; item D does not specify whether the condition of the gas is a standard condition, and the amount of gas substances cannot be calculated. D is wrong. ]
Methods for solving inorganic block diagram inference problems
Inorganic block diagram questions have a small number of words, and the questions and information provided are novel and concise, so it is particularly important to review the questions.
1. The problem-solving steps are generally divided into four steps
(1) Review-read the question carefully and check the meaning of the question clearly. Reading questions include reading and solution requirements. Solving requirements can often provide students with important inspirations, which cannot be ignored.
(2) Find the eye - Find the "eye of the problem", that is, find the breakthrough point to solve the problem, and then combine the information and learned knowledge, and use various thinking methods such as forward and reverse thinking, divergent and convergent thinking, horizontal and vertical thinking, etc., to synthesize Analysis, reasoning. "Looking for the eye" is the key to solving the problem.
(3) Answer - see the requirements clearly and answer them carefully. "Answer" is the starting point of problem solving.
(4) Test-see whether the reasoning is consistent with the meaning of the question. "Check" is the guarantee that the answer is correct.
Among the above steps, the most important thing is to find a breakthrough in solving the problem. Once you find the "eye of the problem", other problems will be easily solved. But don’t ignore the inspection process.
2. There are some ways to find the "question eye" as follows:
(1) Search from the composition and structure of matter. (2) Look for typical properties. (3) Find it from the reaction phenomenon. (4) Find it from the reaction type. (5) Find it from the reaction conditions.
3. Methods for solving inference questions include forward reasoning, backward reasoning, guessing and argumentation, migration, segmentation, enumeration, etc.
Keywords: PPT courseware for high school chemistry compulsory course 1 from the People's Education Press is available for free download, end-of-chapter review lesson PPT download, important elements sodium and chlorine in seawater PPT download, .PPT format;
For more information about the "End of Chapter Review Lesson Important Elements Sodium and Chlorine in Seawater" PPT courseware, please click the "End of Chapter Review Lesson ppt Important Elements Sodium and Chlorine in Seawater ppt" tag.
"End of Chapter Review Lesson" Newton's Laws of Motion PPT:
"End of Chapter Review Lesson" Newton's Laws of Motion PPT Part 1 Content: Consolidation Level Knowledge Integration [Core Quick Filling] 1. The relationship between force and motion: Force can _____ the motion state of an object. 2. Newton's first law: All objects remain at rest or __________..
"End of Chapter Review Lesson" Force and Balance PPT:
"End of Chapter Review Course" Strength and Balance PPT Part One Content: Consolidation Level Knowledge Integration [Core Quick Filling] 1. Synthesis and decomposition of force (1) Obey the rules: _________ rule or _________ rule. (2) The range of the resultant force of two common point forces: _________F_______..
"End of Chapter Review Lesson" interactive PPT:
"End-of-Chapter Review Lesson" Interactive PPT Part One: Consolidation Level Knowledge Integration [Core Quick Filling] 1. The concept of force (1)Vectorality: both ____ and ____. (2) Effect: Make the object ____, change the ____ of the object. 2. Gravity (1)Definition..