"Elements Composing Matter" PPT courseware on the composition of matter Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Elements Composing Matt... | 18975次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Elements Composing Matter" PPT courseware on the composition of matter is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Elements Composing Matter" PPT courseware on the composition of matter, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see







Authoritative PPT Summary
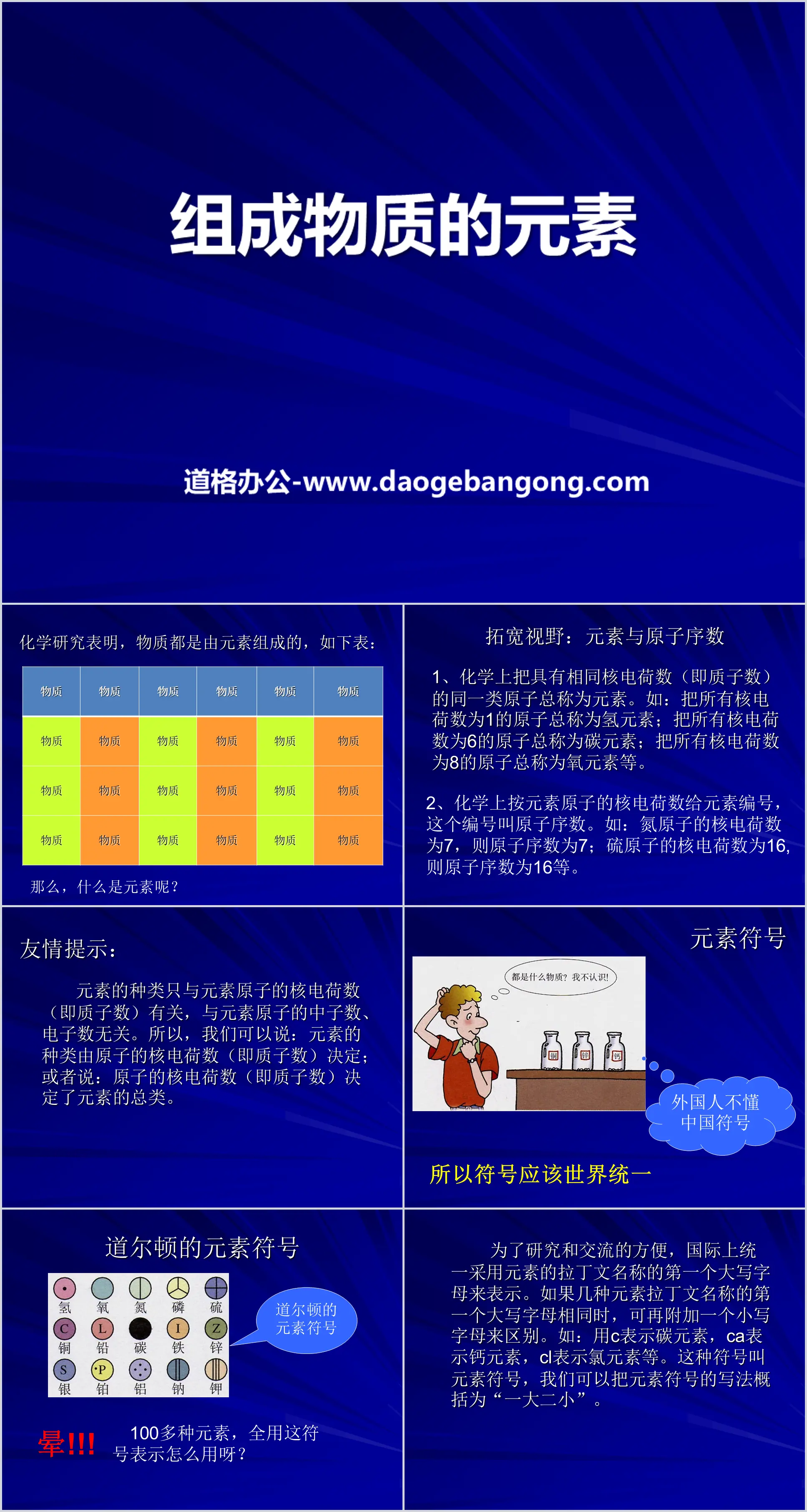
"Elements Composing Matter" PPT courseware on the composition of matter
Broadening your horizons: Elements and atomic numbers
1. In chemistry, atoms of the same type with the same nuclear charge (ie, the number of protons) are collectively called elements. For example: all atoms with a nuclear charge of 1 are collectively called hydrogen elements; all atoms with a nuclear charge of 6 are collectively called carbon elements; all atoms with a nuclear charge of 8 are collectively called oxygen elements, etc.
2. In chemistry, elements are numbered according to the nuclear charge of their atoms. This number is called the atomic number. For example: if the nuclear charge of a nitrogen atom is 7, then the atomic number is 7; if the nuclear charge of a sulfur atom is 16, then the atomic number is 16, etc.
Friendly reminder: The type of element is only related to the nuclear charge number of the element atom (i.e. the number of protons), and has nothing to do with the number of neutrons and electrons of the element atom. Therefore, we can say that the type of element is determined by the nuclear charge of the atom (i.e., the number of protons); or that the type of element is determined by the nuclear charge of the atom (i.e., the number of protons).
What do the element symbols mean?
1. Macroscopic meaning: represents an element;
2. Microscopic meaning: represents an atom of the element.
Friendly reminder: If there is a coefficient other than 1 in front of the element symbol, it can only represent the number of atoms of the corresponding element.
Example 1: “N” represents both nitrogen element and 1 nitrogen atom; 2N can only represent 2 nitrogen atoms
Example 2: Macroscopic meaning: represents oxygen element; microscopic meaning: represents an oxygen atom.
practise:
1. The symbol representing 4 hydrogen atoms is ( )
A. 4H2 B. 4H
C.2H2 D.H4
2. Use chemical symbols to express or tell the meaning of the symbols:
(1) O: __________; __________.
(2) 4S: __________.
(3) n sodium atoms: __________.
(4) One fluorine atom: __________.
Classification of elements
element
Metal elements, such as iron, copper, silver, etc.
Non-metallic elements, such as carbon, oxygen, nitrogen, etc.
Note: Chinese names of elements often include words next to "钅" to represent metallic elements (except "mercury"), and words with "qi" next to "氵" or "石" to represent non-metallic elements.
practise:
Determine which type of elements each of the following elements belongs to:
Fluorine (F) Silver (Ag) Potassium (K) Chlorine (Cl)
Hydrogen (H) Calcium (Ca) Phosphorus (P) Manganese (Mn)
metal element:__________;
Non-metallic elements: __________.
Homework:
1. The essential difference between one element and another element is ( )
A. Different atomic masses B. Different number of protons
C. The number of electrons outside the nucleus is different. D. The number of neutrons is different.
2. Scientists have discovered a new atom with the same number of protons as a hydrogen atom, but one more neutron than a hydrogen atom. The correct statement about this atom is ( )
A. Belongs to a new element
B. Atoms identical to hydrogen atoms
C. is another atom of hydrogen element
D. Atoms of a different type from hydrogen
Keywords: teaching courseware of the composition of matter, teaching courseware of the elements that make up matter, download the Chemistry PPT courseware for the first volume of the ninth grade of the Hunan Education Edition, download the chemistry slide courseware for the ninth grade, download the PPT courseware of the composition of matter, download the PPT courseware of the elements that make up the substance, .PPT format;
For more information about the PPT courseware "Elements Composing Matter and the Composition of Matter", please click the Elements Composing Matter ppt Composition of Matter ppt tag.
"Elements Composing Matter" PPT download:
"Elements Composing Matter" PPT Download Part One: Review Introduction 1. The world is composed of matter. From a microscopic perspective, what is matter made of? It is composed of molecules, atoms, ions and other particles. 2. What is the definition of an element? Have the same nuclear charge..
"Elements Composing Matter" PPT courseware:
"Elements Composing Matter" PPT Courseware Part One: Introduction to Elements What is the definition of an element? Atoms of the same type with the same nuclear charge (or number of protons) are collectively called elements. 1. The world is made of matter. From a microscopic perspective, what does matter consist of?
"Elements Composing Matter" PPT:
"Elements Composing Matter" PPT Part One Content: New lesson introduction to compare the molecular structures of carbon monoxide and carbon dioxide. Conclusion: (1) Both molecules contain carbon atoms and oxygen atoms; (2) The number of oxygen atoms contained in a molecule is different; (3) Both are composed of two elements: carbon and oxygen..