"Diamond, Graphite and C60" Carbon and Carbon Oxides PPT Courseware 4 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Diamond, Graphite and C... | 8475次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Diamond, Graphite and C60" Carbon and Carbon Oxides PPT Courseware 4 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Diamond, Graphite and C60" Carbon and Carbon Oxides PPT Courseware 4, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see










Authoritative PPT Summary
"Diamond, Graphite and C60" Carbon and Carbon Oxides PPT Courseware 4
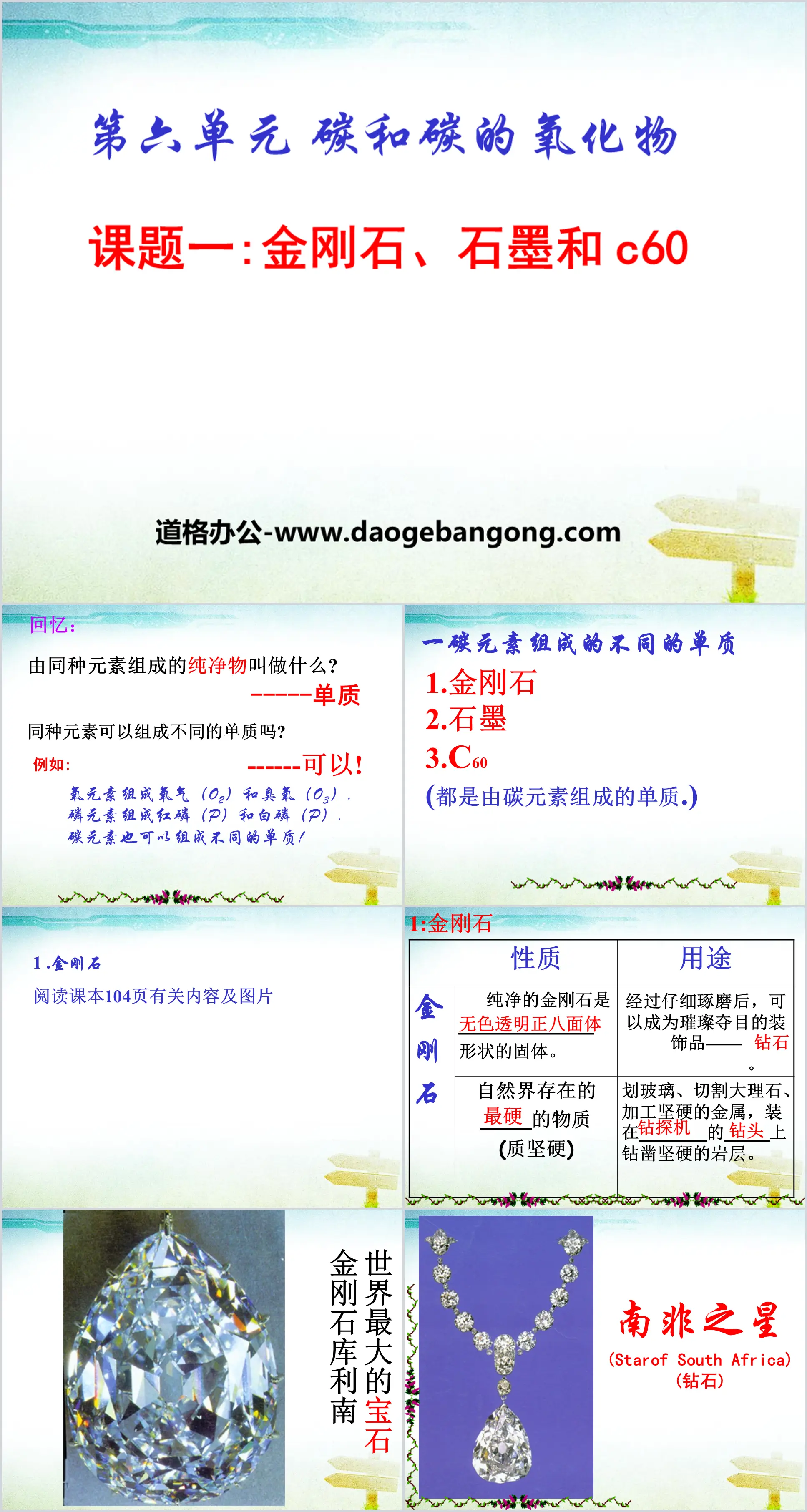
Recall: What is a pure substance composed of the same elements called? -----Elementary substance
Can the same element form different elements? ------Yes!
For example:
The oxygen element consists of oxygen (O2) and ozone (O3),
Phosphorus consists of red phosphorus (P) and white phosphorus (P).
Carbon element can also be composed of different elements!
Different elements composed of one carbon element
1.Diamond
2.Graphite
3.C60
(Both are elements composed of carbon.)
Life tips
Is the lead in a pencil metallic lead?
is a mixture of graphite and clay
HB in pencil:
H stands for Hard, hard;
B stands for Black, black.
6B is the softest and the darkest;
6H is the hardest,
HB has moderate softness and hardness.
If there is a rusty iron lock that has been unused for a long time, how can it be easily opened?
You can put the pencil lead powder into the keyhole, because the pencil lead contains graphite, which has lubricity and can be used as a lubricant.
Related links
Charcoal is mainly composed of tiny crystals of graphite and a small amount of impurities. It has a loose and porous structure, so it has adsorption capacity.
This property of charcoal is used to absorb pigments in some foods and industrial products, and it can also be used to absorb odorous substances.
Coke, activated carbon, carbon black and charcoal have similar structures
Consolidation exercises
1. Connect the relevant items in the following examples on the left and right with lines.
Graphite refrigerator deodorizer, gas mask
carbon black conductive
Diamond new catalyst
C60 ink
Activated carbon cuts hard metal
2. Fill in the following blanks
Elemental substances formed from carbon element mainly include
Graphite, diamond, C60, activated carbon
3. After the discovery of C60, C70 has also been produced. Which of the following statements about C70 is correct ( )
A. It is a compound
B. It is a simple substance, and it is composed of C70 molecules.
C. It is a mixture
D. Its relative molecular mass is 840
4. Which of the following phenomena is a chemical change ( )
A. Activated carbon absorbs colored and toxic gases
B. The pencil leaves the writing on the paper
C. Cutting glass with diamond
D. Graphite burns in air
practice
1. Among the following statements about diamond and graphite, which one is correct ( )
A. Their physical properties are the same
B. They are two different carbon elements
C. They are compounds of two different carbons
D. They are all composed of carbon elements
2. In 1999, NATO used a "graphite bomb". After the bomb exploded, it released a large amount of fibrous graphite to cover the equipment of the power plant and short-circuit the high-voltage transmission lines of Yugoslavia. What properties of graphite were used?
Answer: Taking advantage of the conductivity of graphite
think about it
1. There is a peculiar smell in the water in a small well. Do you have any method to eliminate it?
2. When digging water wells, some charcoal is often buried around the well. Its function is ____________________. This is because charcoal has a __________ structure and therefore has __________ properties.
Basic drill
1. Which of the following substances is definitely a pure substance ( )
(1) Charcoal (2) Substances containing only one element (3) Diamond
(4) Black powder (5) Pencil lead
A.(1)(2)(4) B.(2)(3)(5) C.(2)(3) D.(3)
2. Which of the following statements is correct ( )
A. Diamond and graphite are both elements of carbon.
B. Carbon elements are all black solids
C. Diamond is the hardest of all substances and graphite is the softest.
D. Graphite is a high-quality insulating material
Key points to remember
The main contents that should be mastered in this lesson are:
1. Physical properties and uses of diamond and graphite.
Diamond, hard. Usage: Engraving glass and cutting marble. Drill bit
Conductive Usage: Electrode
Graphite is soft and is used for making pencil leads.
Lubricity Usage: used as lubricant under high temperature conditions
2. The reason for the different physical properties of diamond and graphite: the arrangement of carbon atoms is different.
Types of amorphous carbon: charcoal. Coke. Activated carbon. carbon black
Properties: charcoal. Activated carbon has adsorption properties
Keywords: carbon and carbon oxide teaching courseware, diamond graphite and C60 teaching courseware, People's Education Edition ninth grade chemistry PPT courseware download, ninth grade chemistry slide courseware download, carbon and carbon oxide courseware download, diamond graphite and C60PPT courseware download, .PPT format;
For more information about the PPT courseware "Carbon and Carbon Oxides Diamond Graphite and C60", please click the Carbon and Carbon Oxide ppt Diamond Graphite and C60ppt tag.
"Diamond, Graphite and C60" Carbon and Carbon Oxides PPT Courseware 7:
"Diamond, Graphite and C60" Carbon and Carbon Oxides PPT Courseware 7 What is an element? Can the same element form different elements? Yes, for example: oxygen element can form two different elements, oxygen and ozone, and phosphorus element can form two different elements, red phosphorus and white phosphorus..
"Diamond, Graphite and C60" Carbon and Carbon Oxides PPT Courseware 6:
"Diamond, Graphite and C60" Carbon and Carbon Oxide PPT Courseware 6 Question: Can the same element form different elements? Why? The same element can form different elements. Reason: The same type of atoms, arranged in different ways, form different elements..
"Diamond, Graphite and C60" Carbon and Carbon Oxides PPT Courseware 5:
"Diamond, Graphite and C60" Carbon and Carbon Oxides PPT Courseware 5 Different elements can form different substances. O2 P C Fe Cu H2 N2 Can the same element make up different substances? O2 and O3 Red phosphorus and white phosphorus Substances composed of the same elements must be single...