《Chlorine》Chlorine and its compounds PPT Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| 《Chlorine》Chlorine and... | 2350次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content 《Chlorine》Chlorine and its compounds PPT is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
《Chlorine》Chlorine and its compounds PPT, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)

Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see








Authoritative PPT Summary
《Chlorine》Chlorine and its compounds PPT
Part One: Goals and Competencies:
1. Know the forms of chlorine and that it mainly exists in the ocean in the form of sodium chloride. (Macroscopic identification)
2. Be able to correctly state the physical properties of chlorine. (Macroscopic identification)
3. From the atomic structure characteristics of chlorine and the reaction of chlorine with metals and non-metals, we can understand that chlorine is a non-metallic substance with very active chemical properties. (Macroscopic identification and microscopic analysis)
4. Know the products of the reaction between chlorine gas and water, and be able to correctly list the main components of chlorine water (newly produced chlorine water and long-term chlorine water). (Macroscopic identification and microscopic analysis)
Chlorine PPT, part 2: independent preview to explore new knowledge
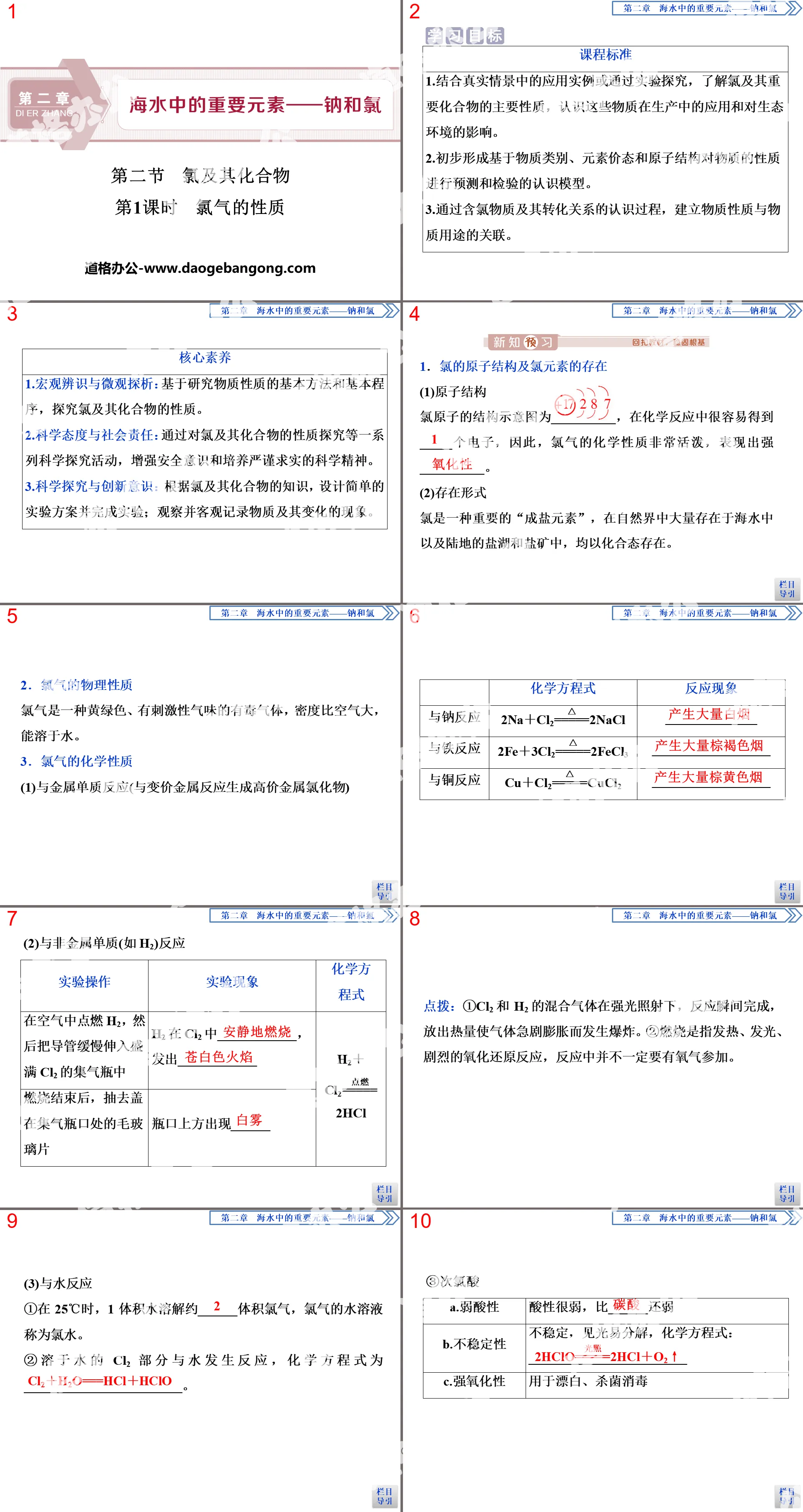
1. Physical properties of chlorine gas
1. Atomic structure and existence of chlorine
2. Physical properties of chlorine gas
2. Chemical Properties of Chlorine
1. Reaction of chlorine gas and metal elements
Write the chemical equation for the following reaction and indicate the main reaction phenomena:
(1) Reacts with sodium to produce a large amount of white smoke;
(2) Reacts with iron to produce a large amount of brown smoke;
(3) Reacts with copper to produce a large amount of brown smoke.
Micro tip: ① Chlorine can react with most metals, and generally oxidizes variable-price metals (such as Fe, Cu) to the highest price. ②Chlorine gas can react with many reducing compounds, such as FeCl2 solution, KI solution, SO2 aqueous solution, etc.
2. Reaction of chlorine gas and non-metallic elemental hydrogen
Ignite hydrogen gas in the air, and then extend the tube into the gas bottle containing chlorine gas.
(1) Phenomenon: Hydrogen ______ quietly in chlorine gas, emitting ______ flame, and ______ appears above the mouth of the gas collecting bottle.
(2)Chemical equation: ____________________.
3. Reaction of chlorine gas and water
(1) Newly produced chlorine water can sterilize and disinfect because chlorine reacts with H2O. The chemical equation of the reaction is ____________. Hypochlorous acid has strong oxidizing properties and can sterilize and disinfect.
(2) Hypochlorous acid
① Hypochlorous acid is a weak acid that is ______ different from water and ______ more acidic than carbonic acid. It cannot be broken down into ionic form when writing ionic equations. Ionic equation for the reaction of chlorine gas and water: ____________________________.
Chlorine gas PPT, the third part: core breakthroughs and difficult difficulties
Composition, properties and applications of chlorine water
1. Chlorine water ingredients
(1) Chemical reactions in chlorine water and ionization of substances
Cl2+H2O===H++Cl-+HClO, H2O��H++OH-, HClO��H+ClO-, 2HClO=====Light 2HCl+O2↑.
(2) The composition of chlorine water - "three molecules and four ions"
2. Properties of chlorine water
Chlorine water contains a variety of particles, so when it reacts with different substances, it exhibits different particle properties, as listed below:
[Typical example] Chlorine water contains a variety of components, so it has a variety of properties. Fill in the blanks according to the reactions of chlorine water with the four substances in the figure (the overlapping parts of a, b, c, and d represent reactions between substances, and the chlorine water is sufficient quantity).
(1) What can prove that chlorine water has bleaching properties is ________ (fill in “a”, “b”, “c” or “d”).
(2)The phenomenon in process c is_______________________________,
The ionic equation for the reaction in process b is ____________________.
(3)The chemical equation of the reaction in process a is ____________________.
Chlorine PPT, Part 4: Meeting standards in class and improving literacy
1. The schematic diagram of the atomic structure of chlorine element is . The following statement is correct ()
A. Chlorine atoms easily lose electrons during chemical reactions
B. Chlorine atoms can easily obtain electrons to form stable chlorine ions
C. The chemical valence of chlorine is only -1
D. Chlorine has 7 electrons outside its nucleus
B: There are 7 electrons in the outermost layer of the chlorine atom. It is easy to obtain 1 electron to form a chloride ion with a stable structure. B is correct. ]
2. Which of the following statements about chlorine is correct ()
A. Sodium burns in chlorine to produce white smoke
B. Red hot copper wire burns in chlorine to form CuCl
C. Pure hydrogen burns quietly in chlorine with a yellow flame
D. Injecting chlorine gas into vole holes to kill voles takes advantage of the toxic and dense properties of chlorine gas
D. Sodium in A burns in chlorine to produce small solid particles of NaCl, forming white smoke. No fog appears (fog is small droplets). A is wrong. The red-hot copper wire in B burns in chlorine to produce CuCl2. , B is wrong; hydrogen in C burns in chlorine and emits a pale flame, C is wrong; chlorine in D is poisonous, denser than air and can kill voles, D is correct. ]
3. The following chlorides can be produced by the direct combination of metals and chlorine, or by the reaction of metals and hydrochloric acid ()
A. CuCl2 B. FeCl2
C. NaCl D. FeCl3
C. Item A, copper does not react with hydrochloric acid, incorrect; item B, iron reacts with chlorine to form ferric chloride, and reacts with hydrochloric acid to form ferrous chloride, incorrect; item C, sodium reacts with chlorine or hydrochloric acid to form both Sodium chloride, correct; item D, according to the analysis in B, D is wrong. ]
Keywords: PPT courseware for high school chemistry compulsory course 1 from the People's Education Press is available for free download, chlorine gas PPT download, chlorine and its compounds PPT download, .PPT format;
For more information about the PPT courseware "Chlorine and its Compounds Chlorine Gas", please click on the Chlorine and Its Compounds ppt Chlorine Gas ppt tag.
"Chlorine and its Compounds" Important elements in seawater - sodium and chlorine PPT (Lesson 2 Laboratory preparation method of chlorine gas and test of chloride ions):
"Chlorine and Its Compounds" Important Elements Sodium and Chlorine in Seawater PPT (Laboratory Method for Preparing Chlorine and Testing of Chloride Ions in Lesson 2) Part One Content: Learning Objectives Course Standards 1. Be able to select experimental devices according to the reaction principle to prepare chlorine gas. 2. Preparation of chlorine gas through laboratory..
"Chlorine and its Compounds" Important elements in seawater - sodium and chlorine PPT (Lesson 1 Properties of chlorine):
"Chlorine and Its Compounds" Important Elements Sodium and Chlorine in Seawater PPT (Properties of Chlorine in Lesson 1) Part One Content: Learning Objectives Course Standards 1. Understand chlorine and its importance through application examples in real situations or through experimental exploration The main properties of compounds, identify...
"Laboratory Preparation Method of Chlorine Gas and Testing of Chloride Ions" PPT courseware on chlorine and its compounds:
"Laboratory Preparation of Chlorine and Testing of Chloride Ions" Chlorine and Its Compounds PPT Courseware Part One Content: Literacy Objectives 1. Review the experimental device of carbon dioxide learned in junior high school and combine it with the manganese dioxide and concentrated hydrochloric acid used to prepare chlorine in the laboratory. Choose appropriate experiments for your properties..