People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Hunan Education Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"Chemical Equations" Chemical Changes and Their Representation PPT Courseware 2
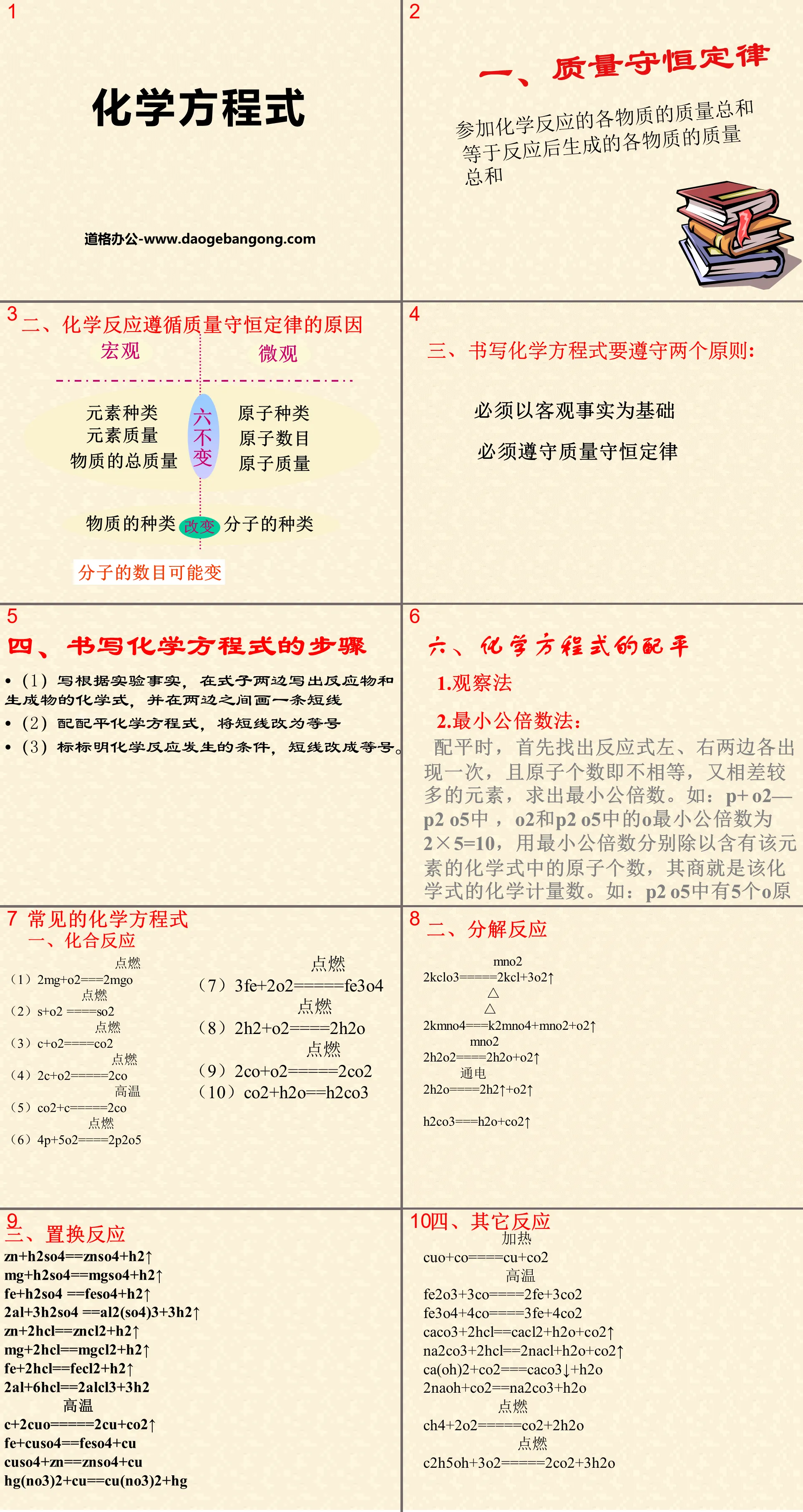
1. Law of conservation of mass
The sum of the masses of all substances participating in a chemical reaction is equal to the sum of the masses of all substances produced after the reaction.
2. Reasons why chemical reactions follow the law of conservation of mass
Type of element Type of atom
Element mass Number of atoms
Total mass of substance Atomic mass
Types of matter Types of molecules
The number of molecules may vary
3. There are two principles to follow when writing chemical equations:
Must be based on objective facts
Must obey the law of conservation of mass
4. Steps to write chemical equations
(1) Based on the experimental facts, write the chemical formulas of the reactants and products on both sides of the formula, and draw a short line between the two sides.
(2) Balance the chemical equation and change the short line into an equal sign
(3) Mark the conditions for the chemical reaction to occur, and change the dashes to equal signs.
Common chemical equations
1. Combination reaction
ignite
(1) 2Mg+O2===2MgO
ignite
(2)S+O2 ====SO2
ignite
(3) C+O2====CO2
ignite
(4) 2C+O2====2CO
high temperature
(5)CO2+C====2CO
ignite
(6)4P+5O2====2P2O5
2. Decomposition reaction
MnO2
2KClO3=====2KCl+3O2↑
2KMnO4===K2MnO4+MnO2+O2↑
MnO2
2H2O2====2H2O+O2↑
power ups
2H2O====2H2↑+O2↑
H2CO3===H2O+CO2↑
5. The meaning of chemical equations (information provided)
a. What substances participate in the reaction (reactants)
b. What substances (products) are generated?
c.Reaction conditions
d. Particle number ratio between reactants and products
e. Mass ratio between reactants and products
9. Calculation steps based on chemical equations
1. Set up the unknown quantity (set up whatever you are asking for, and set up the problem you are asking for at once)
2. Write chemical equations and balance them (chemical equations used in calculations)
3. Write the relative molecular mass, known quantities, and unknown quantities of the relevant substances (only write the substances related to the known and sought, write the relative molecular mass at the top, the mass of the substance at the bottom, and the known units)
4. List proportional formulas and solve them (list direct proportional formulas, the same amount above and below, the same substance on the left and right)
5. Write the answer concisely
Classroom ability training
1. There must be no change before and after the chemical reaction ( )
Number of atoms ② Number of molecules ③ Type of elements ④ Total mass of all substances participating in chemical reactions
⑤Types of matter ⑥Types of atoms
A.①④⑥ B. ①③⑤ C. ①③④⑥ D. ①③④⑤⑥
2. The law of conservation of mass reveals ( ) in chemical reactions
A. Reaction conditions B. Which substances react
C. Which substances are products D. The mass relationship between reactants and products
3. Which of the following statements conforms to the law of conservation of mass ( )
A. Water is heated and turns into water vapor. After the change, the mass is equal.
B.50mL water mixed with 50ml alcohol is not equal to 100mL
C. Contains 10g hydrogen and 80g oxygen in 90g water
D. 1g of sulfur combines with 1g of oxygen to form 2g of sulfur dioxide.
summary
1. Calculations based on chemical equations are based on:
Basis: direct proportional relationship
2. The computational steps for calculating chemical equations are:
①Assume (unknown quantity)
② Square (equation)
③ Off (known or unknown relative molecular mass of related substances)
④Solving ratio (column proportional formula)
⑤Answer (concise answer)
Keywords: Chemical Changes and Their Representation Teaching Courseware, Chemical Equations Teaching Courseware, Hunan Education Edition Ninth Grade Chemistry PPT Courseware Download, Ninth Grade Chemistry Slide Courseware Download, Chemical Changes and Their Representation PPT Courseware Download, Chemical Equation PPT Courseware Download, .PPT format;
For more information about the "Chemical Equations, Chemical Changes and Their Representations" PPT courseware, please click on the Chemical Equations ppt Chemical Changes and Their Representations ppt tag.
"Metal Materials" Iron Metal Materials PPT (Application of the quantity of matter in the calculation of chemical equations in Lesson 2):
"Metal Materials" Iron Metal Materials PPT (Application of the amount of matter in the calculation of chemical equations in Lesson 2) Part One Content: Learning Objectives Course Standards 1. Combined with chemical equations to understand the amount of matter, molar mass, gas molar volume, and matter Concepts such as quantity and concentration...
"Application of the Amount of Substance in the Calculation of Chemical Equations" Metal Materials PPT Download:
"The Application of the Amount of Substance in the Calculation of Chemical Equations" Metal Materials PPT Download Part One Content: Literacy Objective 1. Review and review the relationship between the quantity of substance n and the number of particles N, the mass of the substance m, the gas volume V, and the solution concentration c The calculation formula consolidates the amount of substance as...
"The Application of the Amount of Substance in the Calculation of Chemical Equations" Metal Materials PPT Courseware:
"The Application of the Amount of Substance in the Calculation of Chemical Equations" Metal Materials PPT Courseware Part One Contents: Foundation of Essential Knowledge Literacy 1. Relationship between the Amount of Substance and Each Physical Quantity 1. Illustrated Relationship 2. Calculation Formula (1) has been Know the mass of the substance: n(B)=_______; ..
File Info
Update Time: 2024-11-22
This template belongs to Chemistry courseware Hunan Education Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Chemical Equations" Chemical Changes and Their Representation PPT Courseware 2 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Chemical Equations" Chemical Changes and Their Representation PPT Courseware 2 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Chemical Equations" Chemical Changes and Their Representation PPT Courseware 2, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview