"Chemical Formula and Valence" Water in Nature PPT Courseware 6 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Chemical Formula and Va... | 9050次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Chemical Formula and Valence" Water in Nature PPT Courseware 6 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Chemical Formula and Valence" Water in Nature PPT Courseware 6, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see










Authoritative PPT Summary
"Chemical Formula and Valence" Water in Nature PPT Courseware 6
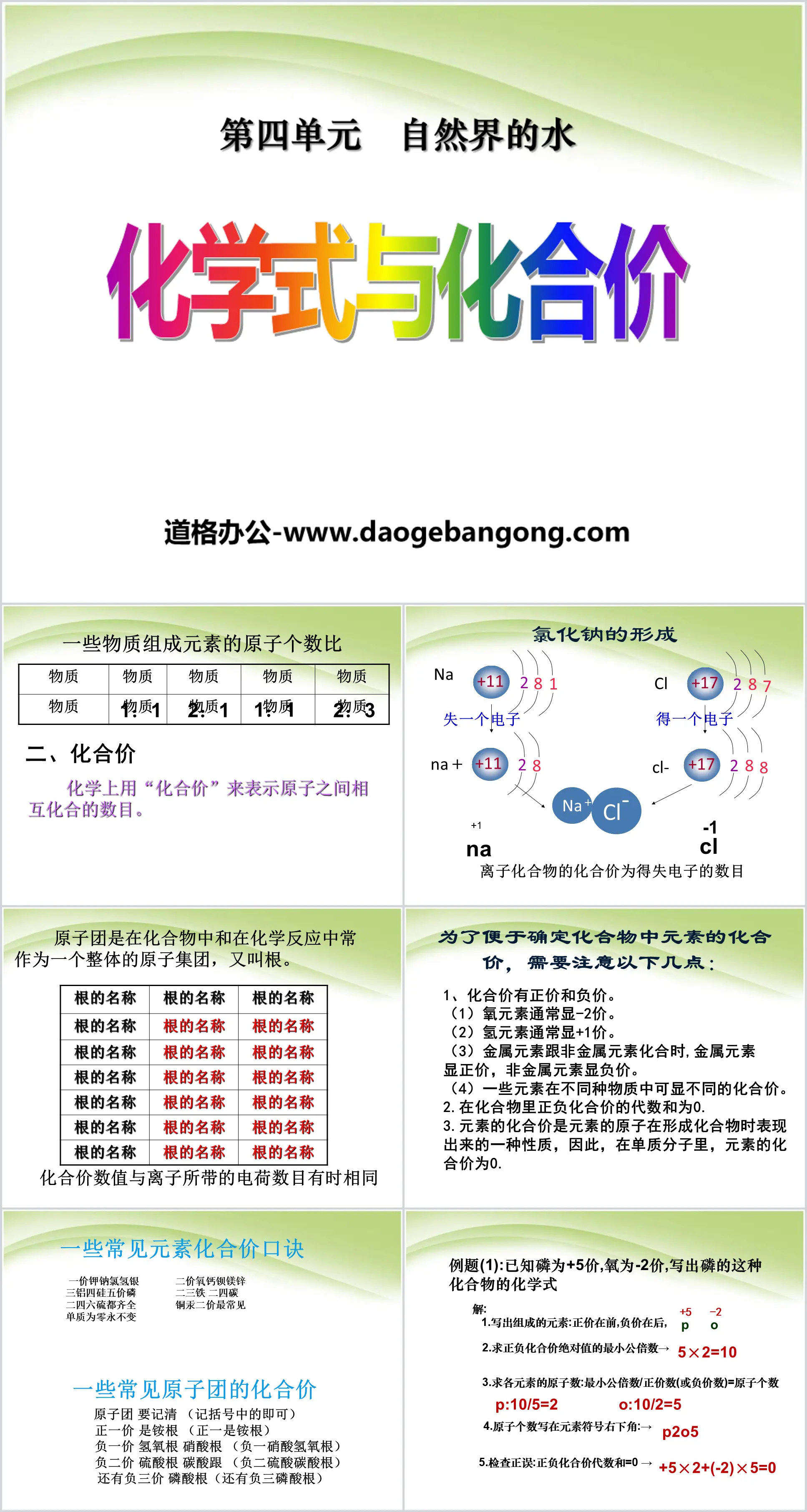
2. Compound price
In chemistry, "valency" is used to represent the number of mutual combinations between atoms.
An atomic group is a group of atoms that often acts as a whole in compounds and chemical reactions, also called roots.
In order to easily determine the valency of elements in a compound, the following points need to be noted:
1. There are positive and negative price of chemical compounds.
(1) Oxygen usually has a valence of -2.
(2) Hydrogen element usually has a valence of +1.
(3) When metal elements are combined with non-metal elements, the metal elements show positive valence and the non-metal elements show negative valence.
(4) Some elements can have different valencies in different substances.
2. The algebraic sum of positive and negative valences in a compound is 0.
3. The valence of an element is a property displayed by the atoms of the element when forming a compound. Therefore, in a single substance molecule, the valence of an element is 0.
practise
Try to write the chemical formula of the following substances
It is known that Na has a valence of +1 and O has a valence of -2. Write their chemical formulas.
It is known that Fe has a valence of +3 and O has a valence of -2. Write their chemical formulas.
It is known that Al has a valence of +3 and Cl has a valence of -1. Write their chemical formulas.
It is known that Mg has a valence of +2 and O has a valence of -2. Write their chemical formulas.
Cross method: When the absolute values of positive and negative valencies of two elements in a compound are not equal, the ratio of the number of atoms is opposite to the absolute value of their valencies.
1. Write the symbol P O
2. Standardized valence P O
3. The absolute value of the chemical valence is placed crosswise in the lower right corner of the element symbol P2 O5
4. Simplify
5. Check
To write a chemical formula based on the valence of elements, follow the following steps:
Two prices are listed in a row. The prices are crossed and written on the lower right.
Reduce the price to a dime. Check if the total price is zero.
practise:
It is known that Al has a valence of +3 and O has a valence of -2. Write their chemical formulas.
It is known that C has a valence of +4 and O has a valence of -2. Write their chemical formulas.
It is known that Ca has a valence of +2 and OH has a valence of -1. Write their chemical formulas.
It is known that Mg has a valence of +2 and SO4 has a valence of -2. Write their chemical formulas.
Three: Calculate the valence based on the chemical formula
Example (2): Given that O has a valence of -2, find the valence of the iron element in Fe2O3.
Solution: Let the valency of iron element in Fe2O3 be X
2X+(-2)×3=0
X=+3
Exercise: Given that SO4 has a valence of -2, find the valence of the iron element in FeSO4.
The valency of variable valence elements must be determined based on calculations.
Four: How to read chemical formulas: read from right to left
A substance composed of two elements is read as "a certain chemical"
Such as: CaCl2 MgO
A substance composed of non-metallic elements and oxygen elements, pronounced as "a few oxides a few"
Such as: P2O5 SO2
A substance composed of metallic elements and atomic groups, pronounced as "a certain acid"
Such as: CaCO 3 CuSO4 NaNO 3
A substance composed of hydrogen atoms and atomic groups is pronounced "a certain acid".
Such as: H2CO3 H2SO4
A substance composed of metallic elements and hydroxyl radicals, pronounced as “hydrogen oxide”
Such as: NaOH Ca(OH) 2 Cu(OH) 2 Fe(OH) 2
Notice:
When it contains "Fe" or "Cu", the valence must be marked before naming to determine whether to add "sub".
Such as: Fe +2 +3
Cu +1 +2
When the price is high, it is read as "iron or copper";
At low prices it is read as "ferrous iron or copper".
Summary of this topic
After studying this topic you should know:
1. The formula that uses element symbols to express the composition of matter is called a chemical formula.
2. The meaning of chemical formula:
Macro: represents a substance; represents the elemental composition of a substance.
Microscopic: represents a molecule; represents the composition of a molecule of a substance.
3. How to read and write chemical formulas
4. Valencies of some common elements and roots
Keywords: Water in nature teaching courseware, chemical formula and valence teaching courseware, PPT courseware download for the first volume of the ninth grade chemistry of the People's Education Edition, download of the ninth grade chemistry slide courseware, water in nature PPT courseware download, chemical formula and valency PPT courseware download, .PPT Format;
For more information about the "Chemical Formula and Valence of Water in Nature" PPT courseware, please click on the "Chemical Formula and Valence of Water in Nature ppt ppt" tab.
"Chemical Formula and Valence" Water in Nature PPT Courseware 7:
"Chemical Formula and Valence" Water in Nature PPT Courseware 7 1. What is a chemical formula 1. The concept of chemical formula: a formula that uses element symbols and numbers to express the composition of matter. (A substance corresponds to a chemical formula) 2. The meaning of chemical formula: Distinguish the number in front of the symbol..
"Chemical Formula and Valence" Water in Nature PPT Courseware 5:
"Chemical Formula and Valency" Water in Nature PPT Courseware 5 1. Chemical Formula 1. Definition: A formula that uses a combination of element symbols and numbers to express the composition of a substance is called a chemical formula. For example: the chemical formula of water is H2O, the chemical formula of oxygen is O2, and the chemical formula of carbon dioxide is The chemical formula is CO..
"Chemical Formula and Valence" Water in Nature PPT Courseware 4:
"Chemical Formula and Valence" Water in Nature PPT Courseware 4 Review: What is an element? What to express? A general term for atoms of the same type with the same nuclear charge, represented by element symbols. What is the meaning of element symbols? It can represent both an element and...