"Chemical Bonding" Periodic Law of Elements in Material Structure PPT (Lesson 2 Covalent Bonding) Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Chemical Bonding" Perio... | 7750次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Chemical Bonding" Periodic Law of Elements in Material Structure PPT (Lesson 2 Covalent Bonding) is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Chemical Bonding" Periodic Law of Elements in Material Structure PPT (Lesson 2 Covalent Bonding), due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)

Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see








Authoritative PPT Summary
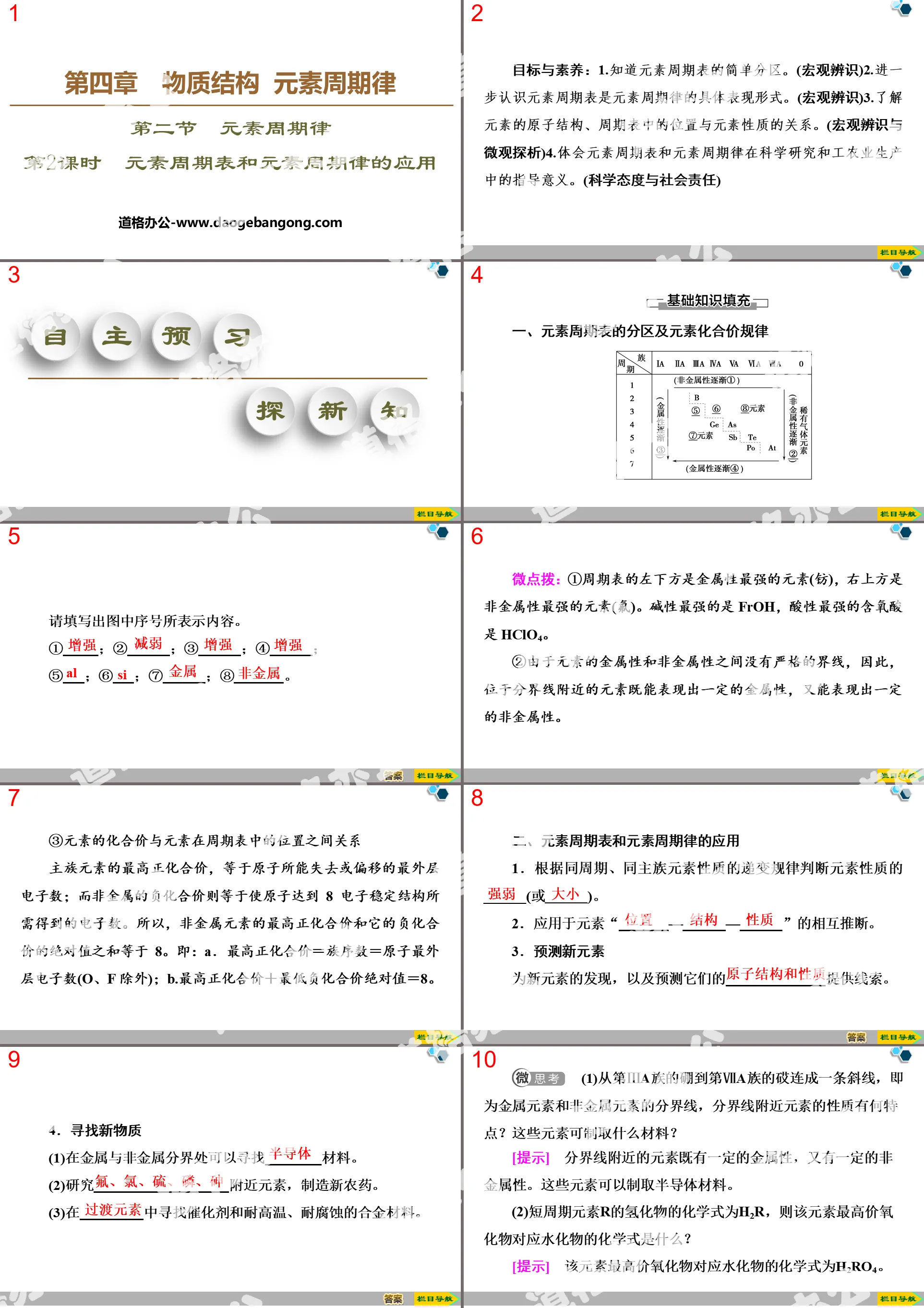
"Chemical Bonding" Periodic Law of Elements in Material Structure PPT (Lesson 2 Covalent Bonding)
Part One: Learning Objectives
Curriculum Standards
1. Understand the interactions between particles that constitute matter, understand the formation of covalent bonds through typical examples, and establish the concept of chemical bonds.
2. Able to determine the types of chemical bonds in simple covalent compounds.
3. Understand that the breaking and forming of chemical bonds is the essence of material changes in chemical reactions.
core competencies
1. Macroscopic identification: Able to use models, symbols and other methods to comprehensively represent the structure and changes of matter.
2. Concept of change: Ability to describe and illustrate the nature and laws of material transformation using macroscopic, microscopic, symbolic and other methods.
Chemical bonding PPT, part 2 content: Knowledge point 1 Covalent bonds and covalent compounds
1. covalent bond
(1) Formation process
①The formation process of chlorine molecules
Two chlorine atoms each provide one electron → a shared electron pair is formed between the two chlorine atoms → both chlorine atoms reach 8e-stable structure → a stable chlorine gas molecule is formed
(2) Covalent bond: The interaction between atoms through _______________.
① Bonding particles: atoms.
②Bonding elements: Generally non-metallic elements of the same or different types.
③ Bonding conditions: The outermost electrons of the atom have not reached a stable state before bonding.
(3)Classification
2. covalent compound
(1) Concept: Compounds that form molecules with ______________.
(2) Four common covalent compounds
①Non-metal hydrides, such as NH3, H2S, H2O, etc.
②Non-metal oxides, such as CO, CO2, SO2, etc.
③ Oxygen-containing acids, such as H2SO4, HNO3, etc.
④Most organic compounds, such as CH4, CH3CH2OH, etc.
3. Representation method and formation process of covalent molecules
(1) Electronic formulas, structural formulas and structural models of common molecules
(2) Use electronic formula to express the formation process of covalent molecules
Troubleshooting
1. Misconceptions about covalent bonds and covalent compounds
(1) Molecules containing covalent bonds are not necessarily covalent compounds, such as H2, O2 and other simple substances.
(2) Compounds containing covalent bonds are not necessarily covalent compounds, such as NaOH, Na2O2, etc.
(3) Ionic compounds may contain covalent bonds, but covalent compounds must not contain ionic bonds, only covalent bonds.
(4) Compounds composed of non-metal elements are not necessarily covalent compounds, such as NH4Cl, etc.
(5) Compounds formed by active metal elements and active non-metal elements are not necessarily ionic compounds. For example, AlCl3 is a covalent compound.
2. Common mistakes in electronic writing of covalent compounds (or elements)
(1) Missing electrons that do not participate in bonding. For example: the electronic formula of N2 is mistakenly written as N⋮⋮N, and should be ••N⋮⋮N••; the electronic formula of NH3 is mistakenly written as , and should be .
(2) The number of shared electron pairs is written incorrectly. For example: the electronic formula of CO2 is mistakenly written as •• and should be ••.
(3) The order of atomic combination is wrong. For example: the electronic formula of HClO is mistakenly written as , it should be .
(4) Wrong use of parentheses. For example: the electronic formula of HCl is mistakenly written as
(5) Confuse the writing of electronic formulas and chemical formulas. For example: the electronic form of H2S is mistakenly written as
use as you learn
1. The figure graphically represents the formation process of hydrogen chloride molecules. Which of the following related statements is incorrect ()
A. Hydrogen chloride is a covalent compound
B. All atoms in the hydrogen chloride molecule have 8 electrons in the outermost shell
C. Hydrogen chloride molecules are more stable than both chlorine atoms and hydrogen atoms
D. Hydrogen chloride molecule contains 1 shared electron pair
2. Which of the following statements is incorrect ()
A. Substances containing covalent bonds must be covalent compounds
B. H2O2 contains both polar and non-polar bonds
C. CaO and NaCl crystals break ionic bonds when they melt
D. Pure sulfuric acid does not conduct electricity
Chemical Bonding PPT, Part 3 Content: Knowledge Point 2 Chemical Bonding and Intermolecular Forces
1. chemical bond
(1) Concept: _____ interaction between adjacent atoms.
(2)Classification
2. Chemical reaction: A process in which atoms in reactants recombine into product molecules. During a chemical reaction, there are _______________ within the reactant molecules and _______________ within the product molecules.
The process of chemical reaction is essentially the process of _______________ and _______________.
3. intermolecular forces
(1) There is a force between molecules that brings the molecules together, which is called intermolecular force. Intermolecular forces were also originally called __________.
(2) Van der Waals forces are much weaker than chemical bonds and mainly affect the melting and boiling points of substances.
4. hydrogen bond
(1) Hydrogen bonding is also an intermolecular force, _____ than chemical bonding, but _____ than van der Waals force.
(2) Hydrogen bonds will _____ the melting point and boiling point of a substance. This is because when a solid melts or a liquid vaporizes, the hydrogen bonds between molecules must be destroyed, which consumes more energy.
use as you learn
1. Which of the following statements about covalent bonds is correct ()
A. Metal atoms can only lose electrons during chemical reactions and therefore cannot form covalent bonds.
B. Molecules formed by covalent bonds can be elemental molecules or compound molecules
C. Covalent bonds can only form between different atoms
D. Only covalent bonds exist in noble gas molecules
2. Which of the following processes does not involve changes in chemical bonds ()
A. quicklime into water
B. Hydrogen chloride gas is introduced into the water
C. ice melts into water
D. Potassium permanganate solid decomposes upon heating
3. When the following substances change, the interactions between particles they overcome are of the same type: ()
A. Liquid bromine and iodine are heated into gases
B. Dry ice and sodium bicarbonate are heated separately to obtain gas
C. Melting of salt and ice
D. Salt and glucose are dissolved in water
Chemical Bonding PPT, Part 4: Qualification Examination Training
1. Which of the following compounds has only covalent bonds ()
A. NaCl B. NaOH
C. (NH4)2SO4 D. H2SO4
2. Which of the following substances are covalent compounds ()
A. Cl2 B. NH4Cl
C. C2H6 D. KOH
3. The correct electronic form of the following substances is ()
4. Which of the following statements about chemical bonds is correct ()
A. Chemical bonds exist between atoms and between molecules
B. The interaction between two atoms is called a chemical bond
C. Ionic bond is the mutual attraction between anions and cations
D. Chemical bonds usually refer to strong interactions between adjacent atoms
Keywords: PPT courseware for high school chemistry compulsory course 1 from the People's Education Press is free to download, chemical bonding PPT downloading, material structure element periodic law PPT downloading, covalent bonding PPT downloading, .PPT format;
For more information about the "Periodic Law of Elements Structure of Materials Chemical Bonds Covalent Bonds" PPT courseware, please click the Periodic Law of Elements ppt Structure of Materials ppt Chemical Bonds ppt Covalent Bonds ppt tag.