People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Cantonese Education Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"Changes of Matter" Everyone Comes to Learn Chemistry PPT Courseware
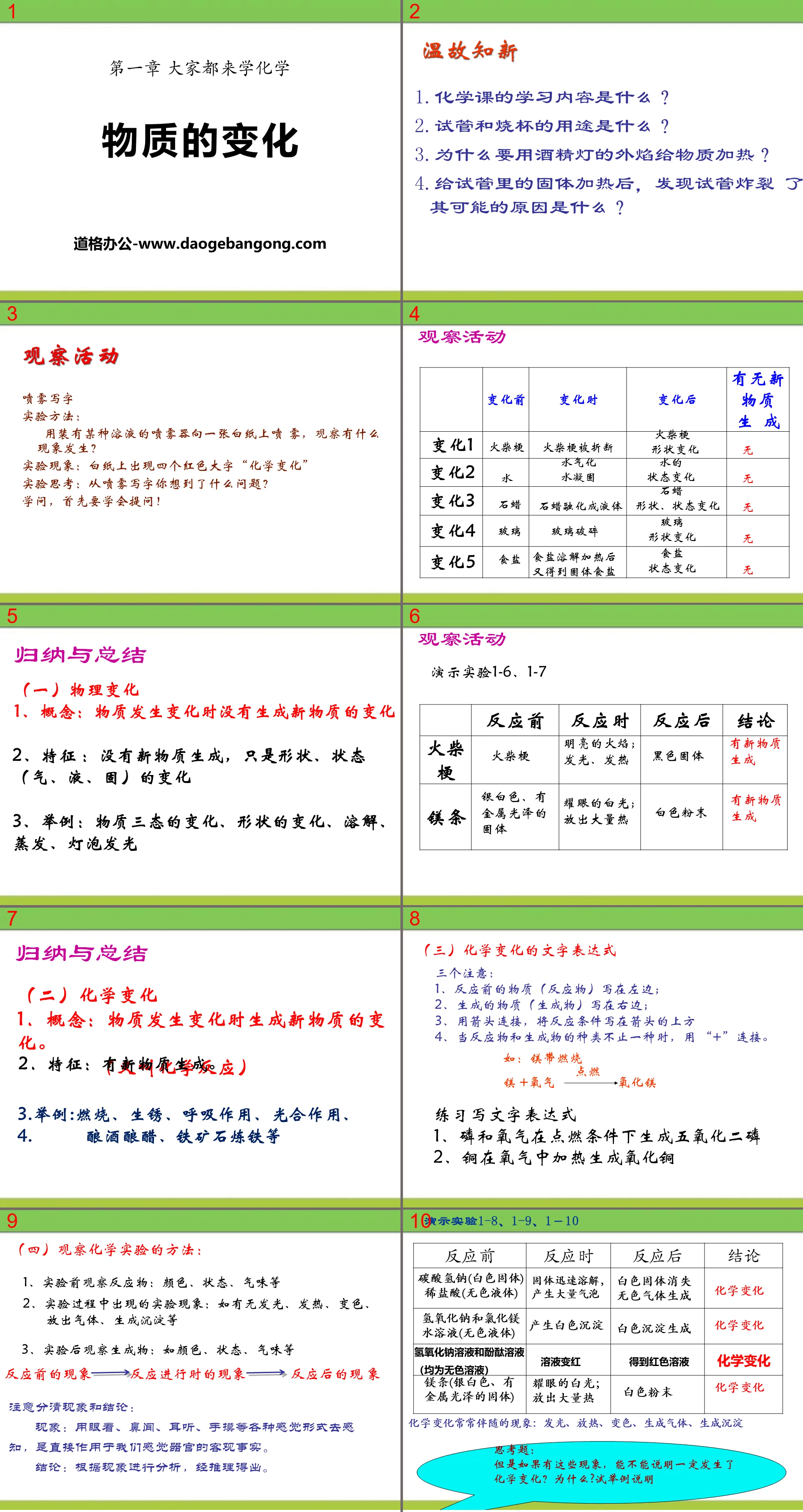
Review the past and learn the new
1. What is the learning content of chemistry class?
2. What are test tubes and beakers used for?
3. Why is it necessary to use the outer flame of an alcohol lamp to heat substances?
4. After heating the solid in the test tube, it was found that the test tube burst. What is the possible reason?
observation activities
spray writing
experimental method:
Use a sprayer filled with a certain solution to spray it on a piece of white paper and observe what happens?
Experimental phenomenon: Four big red characters "Chemical Change" appear on the white paper
Experimental thinking: What issues do you think of when writing with spray?
To learn, you must first learn to ask questions!
Summary and summary
(1) Physical changes
1. Concept: Changes in substances that do not produce new substances when they change
2. Characteristics: No new matter is generated, only changes in shape and state (gas, liquid, solid)
3. Examples: changes in the three states of matter, changes in shape, dissolution, evaporation, and light bulbs
(2) Chemical changes
1. Concept: Changes in substances that create new substances when they change. (also called chemical reaction)
2. Features: New substances are generated.
3. Examples: combustion, rusting, respiration, photosynthesis, wine making, vinegar making, iron ore making, etc.
(3) Text expressions of chemical changes
Three notes:
1. The substances before the reaction (reactants) are written on the left;
2. The generated substances (products) are written on the right;
3. Connect with arrows and write the reaction conditions above the arrows.
4. When there are more than one types of reactants and products, use "+" to connect them.
Practice writing text expressions
1. Phosphorus and oxygen generate phosphorus pentoxide under ignition conditions
2. Copper is heated in oxygen to form copper oxide
(4) Methods of observing chemical experiments:
1. Observe the reactants before the experiment: color, state, smell, etc.
2. Experimental phenomena that occur during the experiment: such as whether there is light, heat, discoloration, gas release, precipitation, etc.
3. Observe the products after the experiment: such as color, state, smell, etc.
Pay attention to distinguishing phenomena and conclusions:
Phenomenon: Perceiving through various sensory modalities such as seeing, smelling, hearing, and touching is an objective fact that directly affects our sensory organs.
Conclusion: Analyze the phenomenon and draw it through reasoning.
Class exercises
1. What kind of changes do the following examples in life belong to? Why?
A broken glass B milk turned sour C copperware rusted D firewood burning
E Glucose brewing F Natural gas burning G Ice and snow melting H Setting off firecrackers
I Tire explosion J Landslide K Evaporation of sea water L Photosynthesis
2. Which of the following changes is a chemical change ( )
A. The light bulb generates heat and glows when it is powered on.
B. Cover the beaker with ice cubes and there will be water droplets on the wall of the beaker.
C. Boiler explosion
D. Cover a beaker above the candle flame with water droplets on the inner wall of the beaker
3. Among the following descriptions of experimental phenomena, which one is correct ( ).
A. After adding sodium hydroxide solution to magnesium chloride solution, magnesium hydroxide precipitates.
B. Carbon dioxide gas is released after the reaction between hydrochloric acid and baking soda.
C. Magnesium strips burn to produce white magnesium oxide
D. When carbon dioxide is passed into clear lime water, the lime water becomes turbid.
Homework
1. What are the characteristics of physical changes and chemical changes?
2. Which of the following phenomena are physical changes and which are chemical changes? Why?
(1) Wet clothes become dry after being exposed to the sun.
(2) Copper forms a patina in humid air.
(3) Paper burning. (4) The porcelain bowl was broken.
(5) Iron rusts. (6) Paraffin wax melts.
(7) Breathing in front of glass in the cold winter will cause a layer of water vapor to appear on the glass.
(8) On a snowy day, put a ball of snow in a warm room and the snow will melt.
3. Textbook pages 19-20 1 and 2
Keywords: Everyone Comes to Learn Chemistry Teaching Courseware, Changes of Matter Teaching Courseware, Guangdong Education Edition Ninth Grade Chemistry PPT Courseware Download, Ninth Grade Chemistry Slide Courseware Download, Everyone Comes to Learn Chemistry PPT Courseware Download, Changes of Matter PPT Courseware Download, .PPT format;
For more information about the "Changes of Matter, Everyone Comes to Learn Chemistry" PPT courseware, please click on the "Changes of Matter ppt, Everyone Comes to Learn Chemistry ppt" tag.
"Material Changes and Us" Material Changes PPT Download:
"Material Changes and Us" Material Changes PPT Download Part One: Import the new lesson and fill it out. 1. The world around us is made of _____, and matter changes. 2. Changes in matter are generally divided into two categories: ____________________. 3...
"Material Changes and Us" Material Changes PPT:
"Material Changes and Us" Material Changes PPT Part One: Material Changes The world around us is made of matter, and matter will change. Changes in matter are generally divided into two categories: physical changes and chemical changes. Chemical changes are accompanied by many phenomena...
"Controlling the Speed of Iron Rusting" PPT teaching courseware on changes in matter:
"Controlling the Speed of Iron Rusting" PPT teaching courseware on changes in matter Part one: Exploration: Is iron rusting related to air? Experimental conclusion: Iron rusting is the result of the combined action of water and air. What does the speed of iron rusting depend on? The speed of iron rusting and the speed of water...
File Info
Update Time: 2024-11-23
This template belongs to Chemistry courseware Cantonese Education Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Changes of Matter" Everyone Comes to Learn Chemistry PPT Courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Changes of Matter" Everyone Comes to Learn Chemistry PPT Courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Changes of Matter" Everyone Comes to Learn Chemistry PPT Courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview