People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Cantonese Education Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
People's Education Press High School Chemistry Compulsory Course 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2 | pptx | 6 MB |
Description
"Understanding Solutions" Solution PPT Courseware
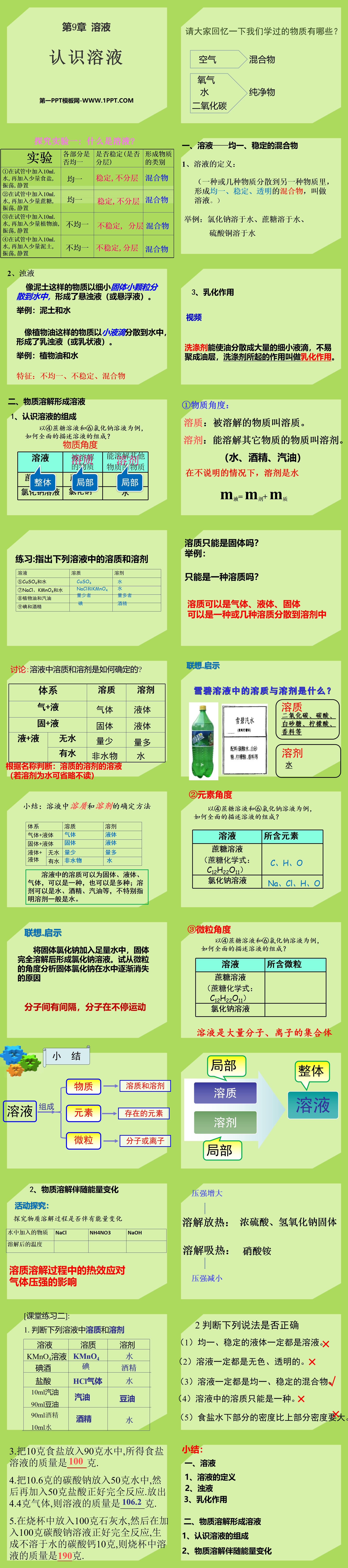
Exploration Experiment 1: What is a solution?
①Add 10mL water into the test tube, then add a small amount of salt, shake and let stand
②Add 10mL water into the test tube, then add a small amount of sucrose, shake and let stand
③Add 10mL water into the test tube, then add a small amount of vegetable oil, shake and let stand
④Add 10mL water into the test tube, then add a small amount of soil, shake and let stand
1. Solution - a homogeneous and stable mixture
1. Definition of solution:
(One or several substances are dispersed into another substance to form a uniform, stable, and transparent mixture, which is called a solution.)
Example: sodium chloride dissolves in water, sucrose dissolves in water, copper sulfate dissolves in water
2. Turbid liquid
Substances like soil are dispersed into water as fine solid particles, forming a suspension (or slurry).
Example: soil and water
Substances like vegetable oil disperse into water in small droplets, forming an emulsion (or emulsion).
Example: vegetable oil and water
Characteristics: Heterogeneous, unstable, mixture
2. Substances dissolve to form solutions
1. Understand the composition of solutions
Taking ④ sucrose solution and ⑥ sodium chloride solution as examples, how to comprehensively describe the composition of the solutions?
①Material perspective:
Solute: The substance that is dissolved is called solute.
Solvent: A substance that can dissolve other substances is called a solvent.
(water, alcohol, gasoline)
Unless otherwise specified, the solvent is water
m liquid = m agent + m substance
②Element angle
Taking ④ sucrose solution and ⑥ sodium chloride solution as examples, how to comprehensively describe the composition of the solutions?
Lenovo. Enlightenment
Add solid sodium chloride to enough water to form a sodium chloride solution after the solid is completely dissolved. Try to analyze the reasons why solid sodium chloride gradually disappears in water from the perspective of particles
There are gaps between molecules, and the molecules are constantly moving
[Class Exercise 2]:
1. Determine the solute and solvent in the following solutions
KMnO4 solution KMnO4 water
iodine iodine alcohol
Hydrochloric acid HCl gas water
10ml gasoline gasoline soybean oil
90ml soybean oil
90ml alcohol alcohol water
10ml water
2. Decide whether the following statements are correct
(1) Uniform and stable liquids must be solutions.
(2) The solutions must be colorless and transparent.
(3) The solutions must be uniform and stable mixtures.
(4) There can be only one type of solute in the solution.
(5) The density of the lower part of the salt water is higher than that of the upper part.
Keywords: solution teaching courseware, understanding solution teaching courseware, Beijing curriculum reform version ninth grade chemistry PPT courseware download, ninth grade chemistry slide courseware download, solution PPT courseware download, understanding solution PPT courseware download, .PPT format;
For more information about the "Solution Understanding Solution" PPT courseware, please click the Solution ppt Understanding Solution ppt tag.
"Understanding Solutions" Solution PPT Courseware 2:
"Understanding Solutions" Solution PPT Courseware 2 Knowledge Review Knowledge Point 1 Solution 1. Concept: refers to a uniform and stable mixture formed by ____________ substances dispersed into ________ substances. 2. Features: Uniformity, stability Uniformity refers to any...
File Info
Update Time: 2024-09-08
This template belongs to Chemistry courseware Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2 industry PPT template
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview