"Atomic Structure" Atomic Structure and Periodic Table PPT Courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Atomic Structure" Atomi... | 15100次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Atomic Structure" Atomic Structure and Periodic Table PPT Courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Atomic Structure" Atomic Structure and Periodic Table PPT Courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)

Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see








Authoritative PPT Summary
"Atomic Structure" Atomic Structure and Periodic Table PPT Courseware
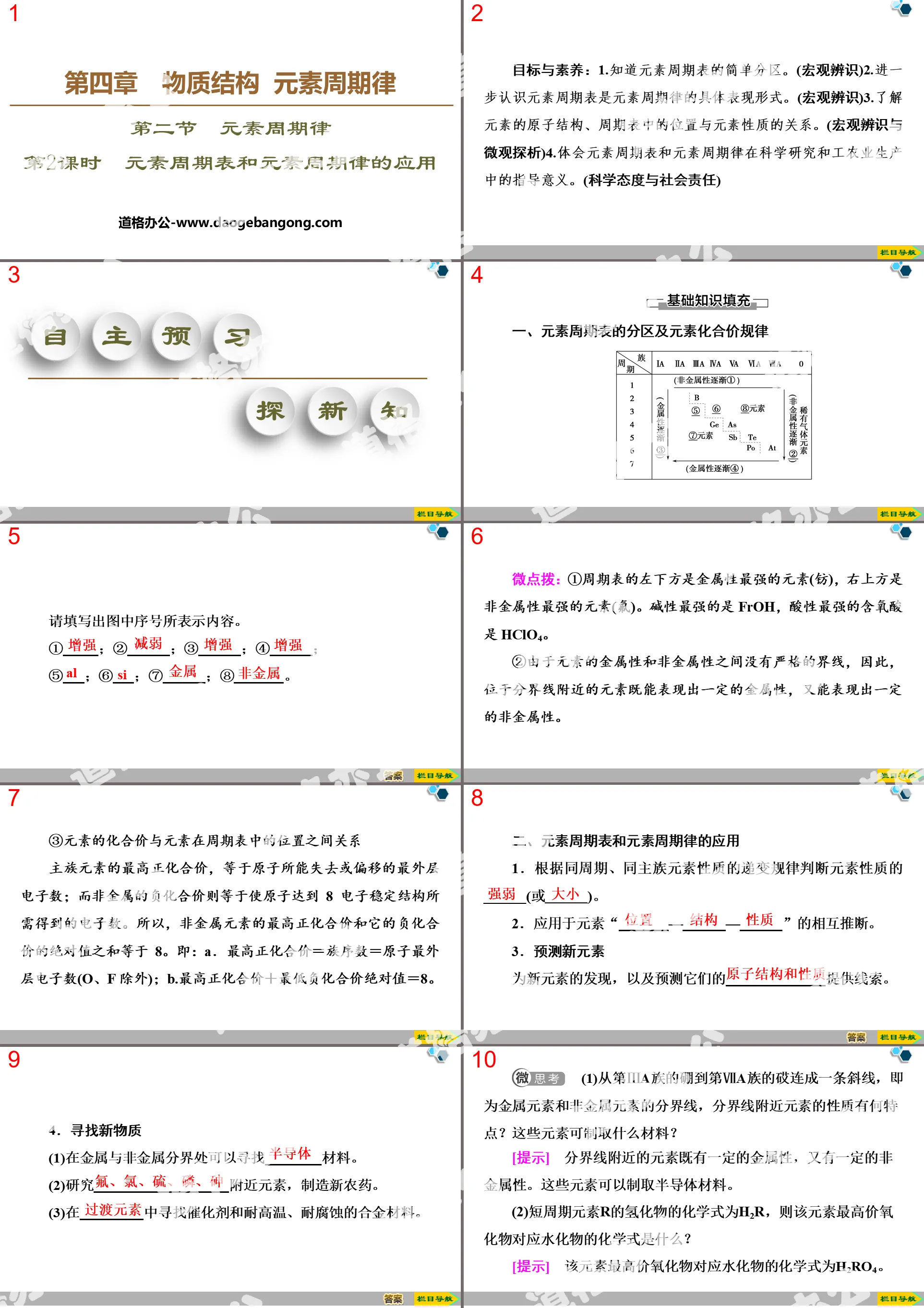
Part 1: Essential knowledge and foundation of literacy
1. The composition of atoms
1. Composition
(2) Relationship: atomic number = nuclear charge = number of protons = number of electrons outside the nucleus (atom is electrically neutral)
[Micro Thoughts] What is the relative mass of a proton or neutron?
Tip: The value obtained by comparing the mass of a proton or neutron with the atomic mass of 12C (1.66×10-27 kg).
2. Mass number
(1) Concept: The relative masses of protons and neutrons are both approximately 1, ignoring electrons
The mass of the atom, approximate the relative masses of all _____ and _____ in the nucleus
Like integer values are added together, and the resulting value is called the mass number.
(2)Relationship: mass number (A) = ____________________
【Smart Judgment】
(1) The number of neutrons in a certain sulfur atom is 18, so its mass number is 34. ()
Tips:√. The number of protons in sulfur is 16. According to the mass number = number of protons + number of neutrons, the mass number of sulfur is 34.
(2) Mass number is the relative atomic mass. ()
Tip:×. The mass number is the approximate integer sum of the relative masses of all protons and neutrons in the nucleus. It is not the same concept as the relative atomic mass.
2. Electronic configuration outside the nucleus
1.Electronic layer
(1) Concept: In a multi-electron atom, the _________ region where electrons move is simplified to _____________, which is called the electron shell.
(2) Representation and energy relationship of different electron layers
【Situation·Thinking】
The onion is a vegetable that we are very familiar with. When the onion is cut, we will see that the inside is presented layer by layer. The electronic layer model is similar to the cut onion. Please think:
(1) Does such a shell really exist around the nucleus?
Tip: The electron layer does not really exist. It is a structural model conceived by scientists based on the areas where electrons often appear in order to express the image.
(2) Do electrons rotate at high speed along a fixed trajectory outside the nucleus?
Tip: Electrons move irregularly in a certain area.
2. Electronic hierarchical arrangement
(1) Principle of minimum energy:
Electrons outside the nucleus are always preferentially arranged in the electron shell of ________, and then
Then they are arranged in the electron layer of _____________ from the inside out, that is, press
K→L→M→N...arranged in order.
(2) The maximum number of electrons that the electron shell can accommodate
①Each electron shell can hold up to ___ electrons. For example, the maximum number of electrons that the K, L, M, and N layers can accommodate are _____________ respectively.
②The number of electrons in the outermost shell cannot exceed __ at most (when the K shell is the outermost shell, it cannot exceed __).
③The maximum number of electrons that can be accommodated in the sub-outer shell does not exceed ___.
Atomic structure PPT, part 2: key abilities and literacy formation
Knowledge point: Hierarchical arrangement of electrons outside the nucleus
[Key points to clarify doubts]
1. The arrangement of electrons outside the nucleus and the relationship between them
2. Representation method of electron arrangement outside the nucleus
(1) Schematic diagram of atomic structure.
①The small circle and the symbols and numbers within the circle represent the atomic nucleus and the number of protons in the nucleus.
②The arc represents the electron layer.
③The numbers within the arc represent the number of electrons in this layer.
(2) Schematic diagram of ion structure.
① When the metal element atoms in the main group lose all their outermost electrons and become ions, the number of electron layers is reduced by one layer, forming the same electron layer structure as the noble gas element atoms in the previous cycle.
② When atoms of non-metal elements gain electrons to form simple ions, they form the same electron layer structure as atoms of rare gas elements with the same period.
【Think·Discussion】
(1) When there are electrons in the M layer, are the K and L layers necessarily full of electrons? When the N layer has electrons, is the M layer full of electrons?
Tip: When there are electrons in the M layer, the K and L layers must be full of electrons. When there are electrons in the N layer, the M layer may not be full.
Tip: If the M layer of a calcium atom arranges 10 electrons, the M layer becomes the outermost layer. This contradicts the rule of electron arrangement that "the number of electrons arranged in the outermost layer cannot exceed 8". It does not It conforms to the rules of electron arrangement, that is, when the M layer is not the outermost layer, it can discharge up to 18 electrons, but when it is the outermost layer, it can only discharge up to 8 electrons.
【Compensation training】
1. (2019·Changsha Grade 1 Test) The hierarchical arrangement of electrons outside the nucleus follows certain quantitative rules and restricts each other. Which of the following statements is definitely wrong ()
A. There is only one electron in the K shell of an atom
B. The number of electrons in the M layer of a certain atom is 4 times the number of electrons in the L layer.
C. The number of electrons in the M layer and L layer of a certain ion is 4 times that of the K layer.
D. The nuclear charge of an atom is equal to the number of electrons in the outermost shell
[Analysis] Choose B. The maximum number of electrons that can be accommodated on the K, L, and M electron shells are 2, 8, and 18 respectively; 1 electron or 2 electrons can be arranged on the K layer, so item A is possible; when there are electrons arranged on the M layer When , 2×4=8, that is, there are M layer and L layer, both of which are 8, so item C is correct; when the K layer serves as the outermost electron layer, the nuclear charge number of the atom is equal to the number of electrons in the outermost layer, such as H, He , so item D is correct.
2. (2019·Zhengzhou Senior High School Test) For an element X with a nuclear charge less than 18, the number of electron shells outside the nucleus is a, and the number of electrons in the outermost shell is (2a+1). Among the following statements about element X, which one is incorrect ()
A. The number of protons in the nucleus of element X is (2a2-1)
B. The element formed by element X can be used as both an oxidizing agent and a reducing agent.
C. A simple ion formed by element X, the number of electrons in each electron shell reaches 2n2 (n represents the number of electron shells)
D. Certain compounds formed from element X may have bactericidal and disinfectant effects
[Analysis] Choose C. Because the number of electron shells cannot be a decimal, it can only be an integer. According to the meaning of the question, 1≤a≤3, and because the number of electrons in the outermost shell is less than or equal to 8, that is, 2a+1≤8, we get 1≤a≤3. When a=1, this is not true; when a=2, the number of electrons in the outermost shell is 5, and element X is N; when a=3, the number of electrons in the outermost shell is 7, and element X is Cl. When X is nitrogen, the number of protons = 2a2-1 = 2×22-1 = 7. When
3.X, Y, and Z are three elements from elements 1 to 18. The number of electrons in the outermost shell of the 2 times, the number of electrons in the sub-outer shell of Z atom is 4 times the number of electrons in the outermost shell. Then the possible combinations of the three elements X, Y, and Z are ()
A.C, Si, MgB.Li, C, Mg
C.C, Mg, Li D.C, O, Mg
[Analysis] Choose A. Among the short-period elements, the element with the number of electrons in the outermost shell is twice the number of electrons in the next outer shell is C. The number of electrons in the second outer shell is twice the number of electrons in the outermost shell, Li and Si. The one with 4 times the number of electrons in the outer shell is Mg, so the answer is option A.
Keywords: PPT courseware for high school chemistry compulsory course 1 from the People's Education Press is available for free download, atomic structure PPT download, atomic structure and periodic table PPT download, .PPT format;
For more information about the "Atomic Structure and Periodic Table of Elements Atomic Structure" PPT courseware, please click on the Atomic Structure and Periodic Table of Elements ppt Atomic Structure ppt tag.
"Atomic Structure and Periodic Table of Elements" Periodic Law of Material Structure PPT (Lesson 2 Atomic Structure and Properties of Elements):
"Atomic Structure and Periodic Table of Elements" Periodic Law of Material Structure Elements PPT (Lesson 2 Atomic Structure and Properties of Elements) Part One Content: Learning Objectives Course Standards 1. Understand the properties of alkali metal elements and halogen elements and their placement in the periodic table of elements relationship between the central position. ..
"Atomic Structure and Periodic Table of Elements" Periodic Law of Elements in Material Structure PPT (Lesson 1 Atomic Structure and Periodic Table of Elements Nuclides):
"Atomic Structure and Periodic Table of Elements" Periodic Law of Material Structure Elements PPT (Lesson 1 Atomic Structure Periodic Table Nuclides) Part One Content: Learning Objectives Course Standards 1. Understand the electron arrangement outside the nucleus. 2. Know the structure of the periodic table of elements. 3. Know..
"Atomic Structure and Properties of Elements" Atomic Structure and Periodic Table of Elements PPT Download:
"Atomic Structure and Properties of Elements" Atomic Structure and Periodic Table PPT Download Part One: Literacy Objectives 1. Know the atomic structure and characteristics of alkali metal elements and halogen elements through the atomic structure and atomic radius information in the textbook tables. 2. Through teaching materials..