"Atomic Structure Periodic Table of Elements" Atomic Structure and Periodic Table of Elements PPT Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Atomic Structure Period... | 14350次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Atomic Structure Periodic Table of Elements" Atomic Structure and Periodic Table of Elements PPT is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Atomic Structure Periodic Table of Elements" Atomic Structure and Periodic Table of Elements PPT, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)

Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see








Authoritative PPT Summary
"Atomic Structure Periodic Table of Elements" Atomic Structure and Periodic Table of Elements PPT
Part One: Literacy Goals
1. Review the atomic structure knowledge learned in junior high school, combined with the schematic diagram of the electron layer model, to understand the relationship between the energy level of electrons outside the nucleus and their layered arrangement. Based on the electron shell arrangement of atoms of rare gas elements, think about and summarize the electron arrangement rules outside the nucleus, and learn to write atomic structure diagrams of common simple atoms. Cultivate awareness and habits of micro-analysis, evidence reasoning and model cognition.
2. Combined with the atomic structure diagram, discuss and summarize the arrangement principles and structure of the periodic table of elements, and determine the position of elements in the periodic table based on the atomic number. Cultivate the ability of evidential reasoning and model cognition.
3. By reading "History of Science", understand the "evolution of atomic structure models" and "the development of the periodic table of elements", cultivate a sense of innovation, and enhance the spirit of scientific inquiry and social responsibility.
Atomic structure PPT, part 2: independent preview before class
Knowledge foundation
1. Element is a general term for atoms of the same type with the same nuclear charge (i.e. number of protons).
2. The number of protons in an atom = the number of nuclear charges = the number of electrons outside the nucleus.
3. The atomic structure diagrams of magnesium, aluminum, sulfur and chlorine are respectively
4. What extranuclear electron arrangement structure is a stable structure?
It indicates that the outermost shell has a structure of 8 electrons (2 electrons when there is only 1 electron shell).
Preview of new knowledge
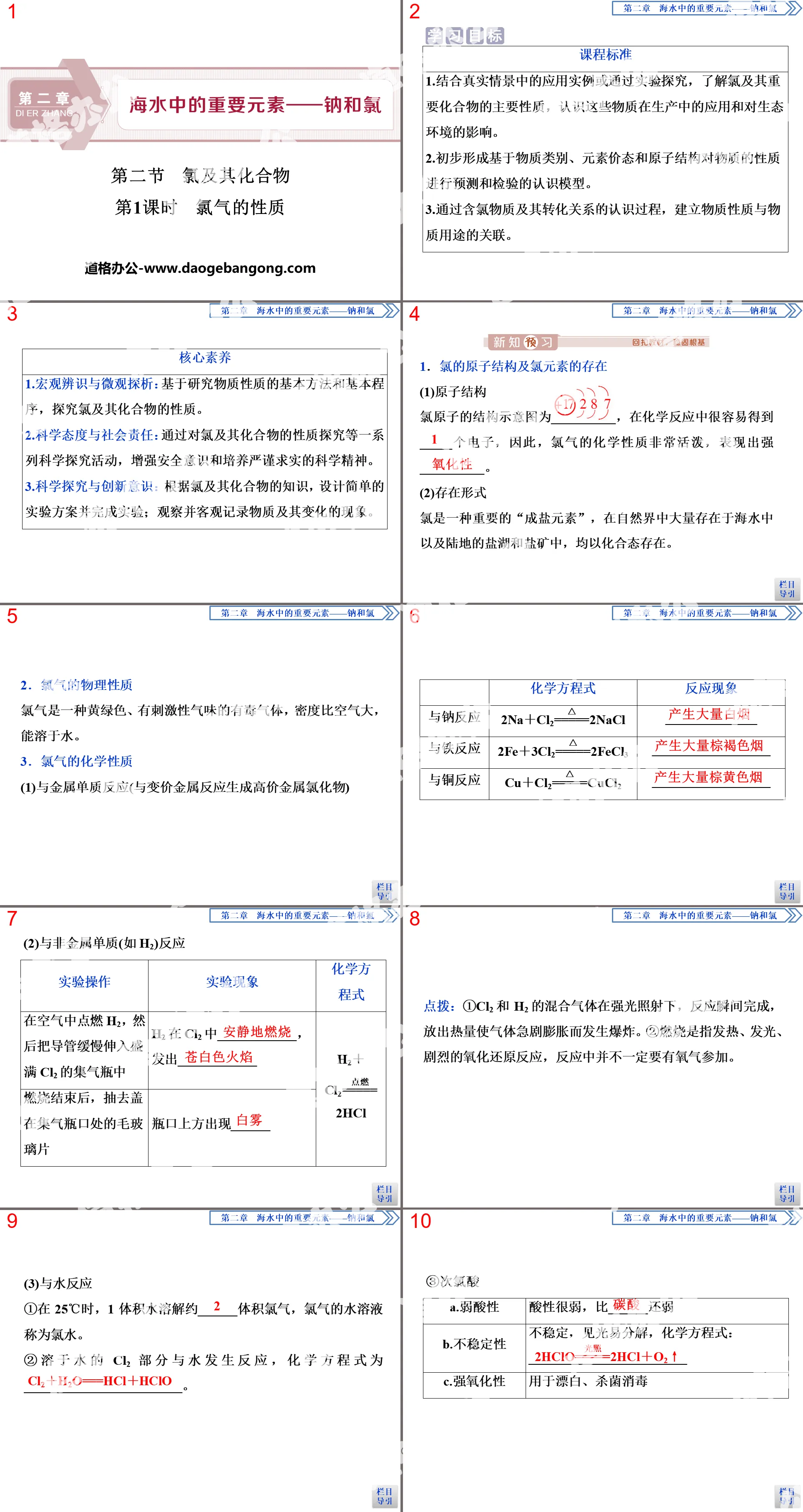
1. Atomic structure
1. The composition of atoms
2. Mass number
(1) Mass number: Add the approximate integer values of the relative masses of all protons and neutrons in the nucleus, and the resulting value is called the mass number.
(2) Equivalent relationship: mass number (A) = number of protons (Z) + number of neutrons (N).
3. The arrangement of electrons outside the nucleus
(1) Division of electronic layers
①The energy and movement area of electrons outside the nucleus.
②Electron shell and its relationship with energy.
(2) The arrangement of electrons outside the nucleus
①Electrons are always discharged from the inner layer as much as possible first, and then fill the next layer when one layer is full.
② Each electron shell outside the nucleus can accommodate up to 2n2 electrons.
③The number of electrons in the outermost shell of an atom cannot exceed 8 (when the K shell is the outermost shell, it cannot exceed 2), the number of electrons in the second outermost shell cannot exceed 18, and the number of electrons in the penultimate layer cannot exceed 32.
4. Representation method of electronic layer
The structural diagram of sodium atom is as follows, please indicate its significance.
[Micro Thought 1] What is the quantitative relationship between the particles that make up atoms or ions?
Tips (1) Atom: nuclear charge = number of protons = number of electrons outside the nucleus.
(2) Cation: Number of protons = number of electrons outside the nucleus of the cation + number of charges of the cation.
(3) Anion: The number of protons = the number of electrons outside the nucleus of the anion - the charge number of the anion.
5. Evolution of atomic structure models
Humanity's understanding of atomic structure has gone through the following evolution process: Dalton model → Thomson atomic model → Rutherford atomic model → Bohr atomic model → electron cloud model, etc.
2. Periodic table of elements
1. The development history of the periodic table of elements
2.Atomic number
(1) Meaning: Number the elements according to their order in the periodic table to get the atomic number.
(2)The relationship between atomic number and atomic structure
Atomic number = nuclear charge = number of protons = number of electrons outside the nucleus
3.Arrangement principles
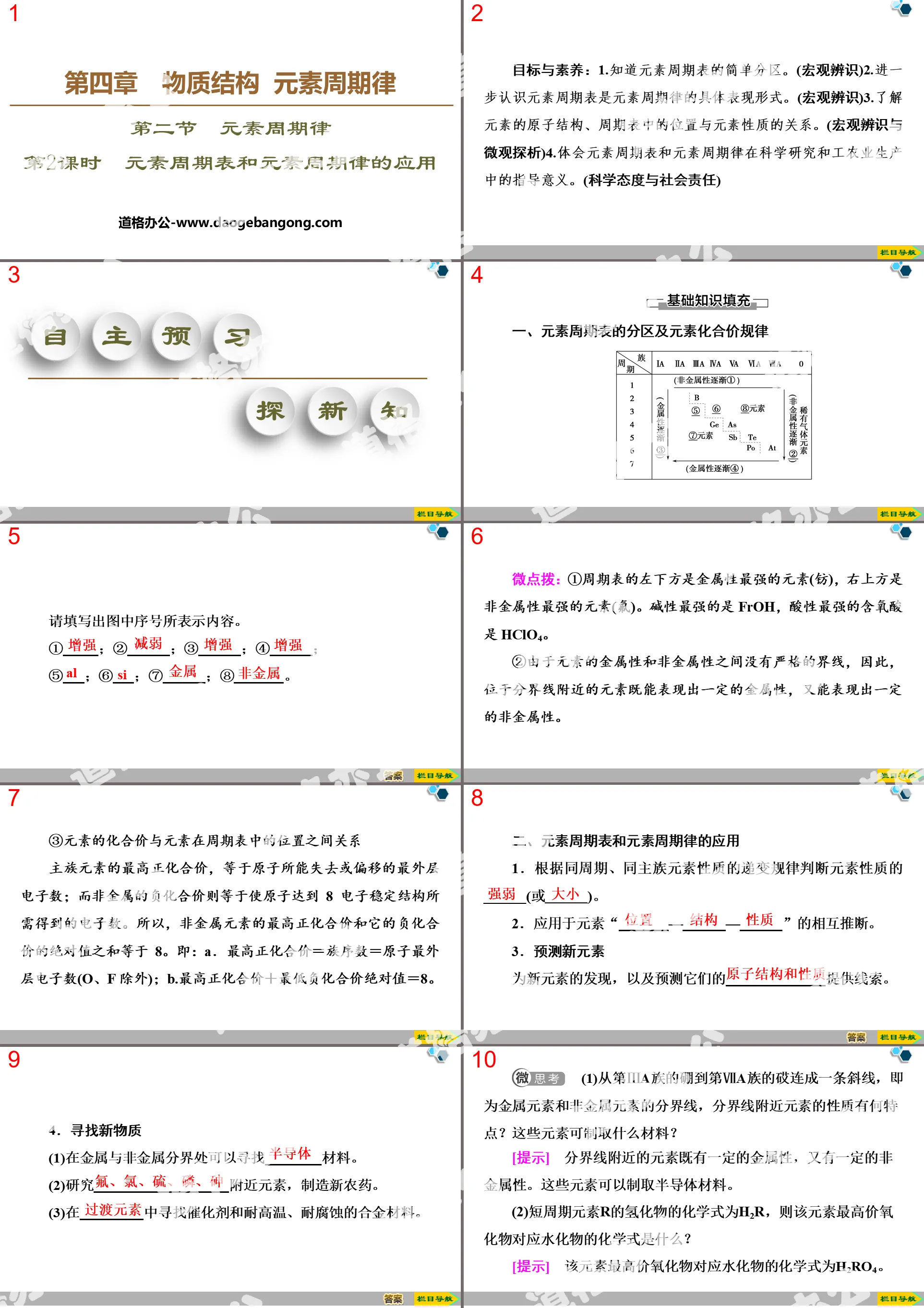
4. Structure of the periodic table of elements
(1) Cycle (horizontal)
①Number: There are 7 periods in the periodic table of elements.
②Features: The number of electron shells of elements in each cycle is the same.
③Classification (3 short, 4 long)
Short cycle: including one, two and three cycles (3 short).
Long cycle: including four, five, six and seven cycles (4 long).
(2) Family (column)
①Number: There are 18 columns in the periodic table of elements, but only 16 groups.
② Features: The group number of the main group element in the periodic table is equal to the number of electrons in its outermost shell.
Autonomous testing
1. Judge whether it is right or wrong (mark “√” if it is correct and “×” if it is wrong).
(1) The electrons outside the nucleus have different energies, and those with higher energy move in areas closer to the nucleus. ()
(2) The M shell of a potassium atom can discharge 9 electrons. ()
(3) The outermost electrons in each cycle range from 1 to 8 electrons. ()
(4) The number of electron shells of elements in the same period must be the same. ()
(5) The number of outermost electrons of elements in the same group must be equal to the group number. ()
(6) The number of electrons in the outermost shell of Group 0 elements is 8. ()
(7) Each column must be a family. ()
(8) The periodic table of elements has developed into a stable form and no new changes are possible. ()
Answer(1)× (2)× (3)× (4)√ (5)× (6)× (7)× (8)×
The position of element 2.15 P in the periodic table is ()
A. Group VA of the second cycle B. Group VIA of the second cycle
C. Group VA of the third cycle D. Group VA of the third cycle
AnswerD
By analyzing and drawing the atomic structure diagram of element No. 15, it can be determined that the position of P element in the periodic table is Group VA of the third period.
3. The following tables are part of the periodic table (the numbers in the table represent the atomic numbers of elements), the correct one is ()
AnswerD
Analysis: Element No. 2 in item A should be in the last column of the periodic table, wrong; No. 2, 3, and 4 in item B should not be in the same period, because there are only two elements H and He in the first period, wrong; item C Elements No. 6, 12 and 24 should not be in the same main group, because the difference in atomic number of main group elements in adjacent periods can only be 2, 8, 18, 32. There is no difference of 6 or 12. Wrong ;Item D completely complies with the arrangement rules of the periodic table of elements.
Atomic structure PPT, the third part: classroom exploration and learning
Electronic arrangement rules and expression methods outside the nucleus
Question exploration
(1) The electron arrangement characteristics outside the nucleus of elements with nuclear charges ranging from 1 to 20. (fill in element symbol)
①Elements with one electron in the outermost shell of the atom:_______________.
②Elements with 2 electrons in the outermost shell of the atom: ______________.
③Elements in which the number of electrons in the outermost shell of an atom is equal to the number of electrons in the next outermost shell: _______.
④Elements in which the number of electrons in the outermost shell of an atom is twice the number of electrons in the next outer shell: _______. Elements in which the number of electrons in the outermost shell of an atom is three times the number of electrons in the next outermost shell: _______. Elements in which the number of electrons in the outermost shell of an atom is 4 times the number of electrons in the next outermost shell: _______.
⑤ Elements in which the number of electrons in the outermost shell of an atom is twice the number of electron shells: _______. Elements in which the number of electrons in the outermost shell of an atom is three times the number of electron shells: _______.
⑥Elements whose atomic number of electron shells is equal to the number of electrons in the outermost shell: _______.
⑦Elements whose atomic number of electron shells is twice the number of electrons in the outermost shell: _______.
⑧Elements whose total number of atomic electrons is twice the number of electrons in the outermost shell: _______.
⑨Elements whose atoms have twice the number of electrons in the outer shell are twice the number of electrons in the outermost shell: _______.
⑩Elements in which the number of electrons in the inner shell of an atom is twice the number of electrons in the outermost shell: _______.
11Atoms without neutrons in the nucleus:_______.
Deepen and expand
1. The arrangement of electrons outside the atomic nucleus - the "four best"
(1) "a minimum"
The electrons outside the nucleus are always arranged in the electron layer with the lowest energy first, and then gradually arrange from the electron layer with lower energy to the electron layer with higher energy from the inside out, that is, the K layer is filled up, then the L layer is filled, and L is filled up The layers are arranged in M layers, and so on.
(2) "Three at most"
①The maximum number of electrons that each electron shell can accommodate is 2n2. For example, the K, L, M, and N layers can hold up to 2, 8, 18, and 32 electrons respectively.
②The maximum number of electrons in the outermost shell is 8 (when the K shell is the outermost shell, the maximum number is 2).
③The maximum number of electrons in the sub-outer shell is 18.
2. Representation method of electron arrangement outside particle core
(1) Schematic diagram of atomic structure: a diagram showing the nuclear charge of an atom and the arrangement of extranuclear electrons in each electron layer outside the nucleus.
For example, the atomic structure diagram of Na atom is
(2) Schematic diagram of ion structure
The symbols in the ion structure diagram have the same meaning as the atomic structure diagram, but note that the number of protons in the atomic structure diagram is equal to the number of electrons outside the nucleus, while the number of protons in the ion structure diagram is not equal to the number of electrons outside the nucleus. like
3. Common particles containing 2e-, 10e-, 18e-
(1) Particles with 2 electrons outside the nucleus: Li+, Be2+, H-, He, H2.
(2) Particles with 10 electrons outside the nucleus
Molecules: CH4, NH3, H2O, HF, Ne
Cation: Na+, Mg2+, Al3+, N, H3O+
Anions: N3-, O2-, F-, OH-, N
(3) Particles with 18 electrons outside the nucleus
Molecules: Ar, HCl, H2S, PH3, SiH4, F2, H2O2, N2H4, etc.
Cation: K+, Ca2+
Anions: P3-, S2-, HS-, Cl-
Atomic structure PPT, part 4: on-site testing
1. Which of the following statements does not conform to the basic rules of electron arrangement outside the nucleus ()
A. Electrons outside the nucleus always prioritize the electron shell with the lowest energy.
B.K layer is the electron layer with the lowest energy
When the C.N electron layer is the sub-outer layer, the maximum number of electrons that can be accommodated is 18
D. The maximum number of electrons that each electron shell (n) can accommodate is n2
AnswerD
Analyze that the maximum number of electrons that the nth electron shell can accommodate is 2n2.
2. Among elements with a nuclear charge less than or equal to 18, the number of electrons in the outermost shell of an atom is half of the total number of remaining electrons: ()
A.1 species B.2 species C.3 species D.4 species
Answer B
Analyze that among elements 1 to 18, the electron configurations of the element atoms that meet the requirements of the question are 2, 1 and 2, 8, 5.
3. Which of the following statements about the periodic table of elements is correct ()
A. Short cycles refer to the first, second, third and fourth cycles
B. The fifth column in the periodic table is group VA
C. The group with the most elements in the periodic table is Group IIIB
D. There are 18 groups in the periodic table of elements
AnswerC
4. There is only one electron in the outermost electron shell of the atom of a certain short-period element. This element ()
A. It must be a metal element
B. It may be a metallic element or a non-metallic element
C. It must be a non-metallic element
D. Must be a non-main group element
Answer B
Analysis Group IA elements have only one electron in the outermost shell, and H is a non-metallic element, while other elements are metallic elements.
5. A, B, C, and D are short-period elements. Their positions in the periodic table are as shown in the figure. The sum of the number of electrons outside the nucleus of elements A and C is equal to the number of protons in the atom of element B.
(1) Write the name of element A: ________.
(2)B is located in the ________ period and group ________ of the periodic table.
(3) The atomic structure diagram of C is ________.
(4)The chemical equation of the reaction between the element D and water is ________.
Keywords: PPT courseware for high school chemistry compulsory course 1 from the People's Education Press is available for free download, atomic structure PPT download, periodic table of elements PPT download, atomic structure and periodic table of elements PPT download, .PPT format;
For more information about the PPT courseware "Atomic Structure and Periodic Table of Elements Atomic Structure of Elements Periodic Table of Elements", please click on the Atomic Structure and Periodic Table of Elements ppt Atomic Structure ppt Periodic Table of Elements ppt tag.
"Periodic Law of Elements" Periodic Law of Elements in Material Structure PPT (Application of the Periodic Table of Elements and Periodic Law of Elements in Lesson 2):
"Periodic Law of Elements" Periodic Law of Elements in Material Structure PPT (Application of the Periodic Table of Elements and Periodic Law of Elements in Lesson 2) Part One Content: Learning Objectives Course Standards 1. Be able to use the position and atomic structure of elements in the periodic table to analyze , prediction, comparison elements..
"Atomic Structure and Periodic Table of Elements" Periodic Law of Material Structure PPT (Lesson 2 Atomic Structure and Properties of Elements):
"Atomic Structure and Periodic Table of Elements" Periodic Law of Material Structure Elements PPT (Lesson 2 Atomic Structure and Properties of Elements) Part One Content: Learning Objectives Course Standards 1. Understand the properties of alkali metal elements and halogen elements and their placement in the periodic table of elements relationship between the central position. ..
"Atomic Structure and Periodic Table of Elements" Periodic Law of Elements in Material Structure PPT (Lesson 1 Atomic Structure and Periodic Table of Elements Nuclides):
"Atomic Structure and Periodic Table of Elements" Periodic Law of Material Structure Elements PPT (Lesson 1 Atomic Structure Periodic Table Nuclides) Part One Content: Learning Objectives Course Standards 1. Understand the electron arrangement outside the nucleus. 2. Know the structure of the periodic table of elements. 3. Know..