"Vaporization and Liquefaction" PPT courseware on changes in the state of matter (Vaporization in Lesson 1) Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Vaporization and Liquef... | 10675次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Vaporization and Liquefaction" PPT courseware on changes in the state of matter (Vaporization in Lesson 1) is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Vaporization and Liquefaction" PPT courseware on changes in the state of matter (Vaporization in Lesson 1), due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see







Authoritative PPT Summary
"Vaporization and Liquefaction" PPT courseware on changes in the state of matter (Vaporization in Lesson 1)
Part One Content: Basic Knowledge Key Points
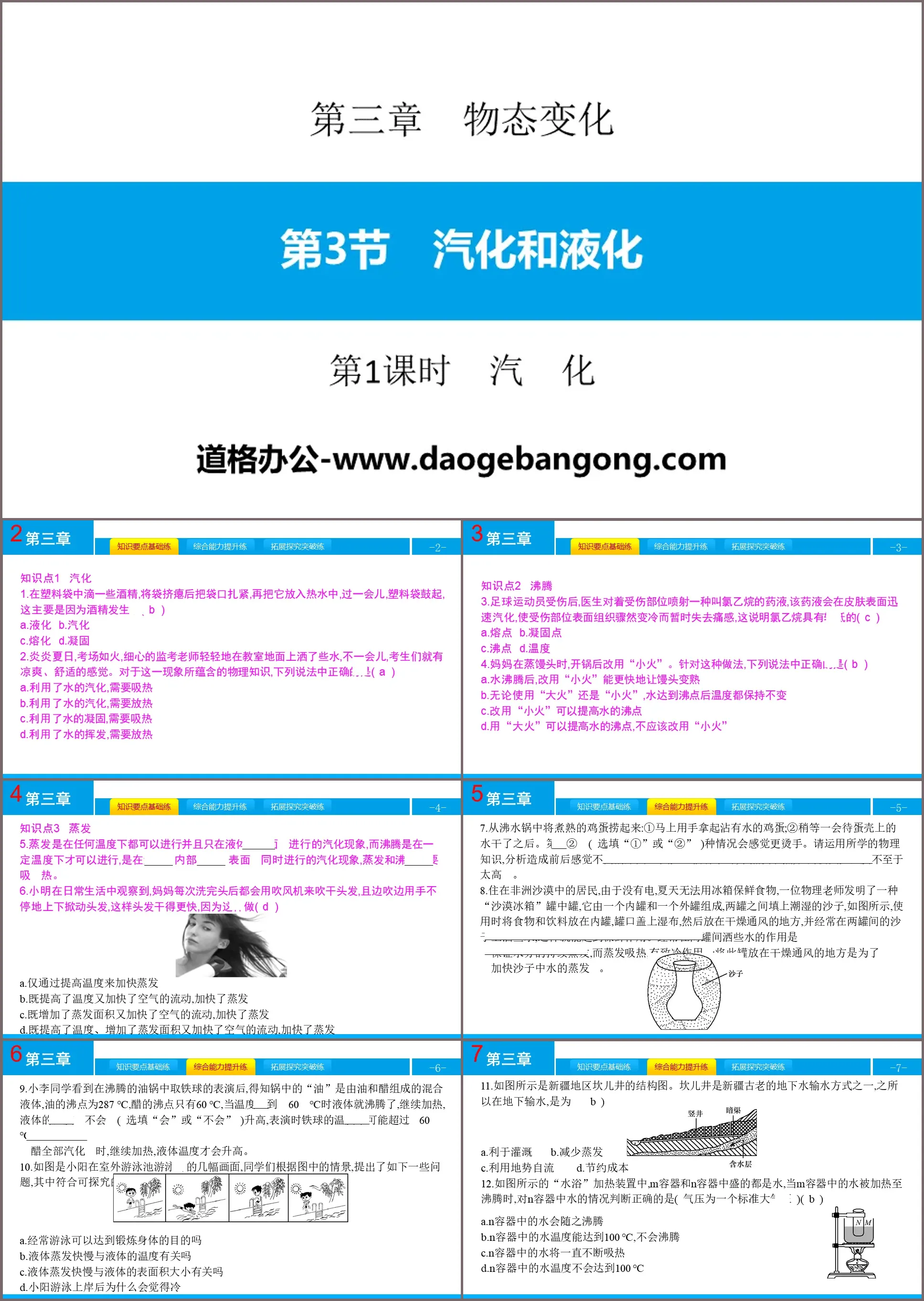
Knowledge point 1 Vaporization
1. Drop some alcohol into a plastic bag, deflate the bag, tie the mouth of the bag tightly, and then put it into hot water. After a while, the plastic bag will bulge. This is mainly because the alcohol has produced (B)
A.Liquefaction B.Vaporization
C. Melting D. Solidification
2. In the hot summer, the examination room was like a fire. The careful invigilator gently sprinkled some water on the classroom floor. After a while, the candidates felt cool and comfortable. Regarding the physical knowledge contained in this phenomenon, which of the following statements is correct (A)
A. Utilizes the vaporization of water and needs to absorb heat
B. Utilizes the vaporization of water and needs to release heat
C. It takes advantage of the solidification of water and needs to absorb heat.
D. Utilizes the volatilization of water and needs to release heat
Knowledge Point 2: Boiling
3. After the football player was injured, the doctor sprayed a liquid called ethyl chloride on the injured area. The liquid would quickly vaporize on the surface of the skin, causing the surface tissue of the injured area to suddenly cool down and temporarily lose the pain. This shows that ethyl chloride Alkanes have lower (C)
A. Melting point B. Freezing point
C. Boiling point D. Temperature
4. When my mother steamed the steamed buns, she switched to "low heat" after boiling the pot. Regarding this approach, which of the following statements is correct (B)
A. After the water boils, switching to "low heat" can make the steamed buns cook faster
B. Regardless of whether you use "high fire" or "low fire", the temperature will remain unchanged after the water reaches the boiling point.
C. Switching to "low heat" can increase the boiling point of water
D. Using "high fire" can increase the boiling point of water, and you should not use "low fire" instead.
Vaporization and liquefaction PPT, Part 2: Comprehensive Capability Improvement
7. Pick up the cooked eggs from the boiling water pot: ① Immediately pick up the water-covered eggs with your hands; ② Wait for a while until the water on the eggshells dries. The first ② (optional “①” or “②”) situation will feel hotter. Please use the physics knowledge you have learned to analyze the reasons for the different feelings before and after: When there is water on the eggshell, the water evaporates and absorbs heat, so that the temperature of the eggshell is not too high.
8. Residents living in the African desert cannot use refrigerators to keep food fresh in the summer due to lack of electricity. A physics teacher invented a "desert refrigerator" jar-in-a-jar, which consists of an inner jar and an outer jar. Fill the space with moist sand, as shown in the picture. When using, put food and drinks in the inner can, cover the mouth of the can with a damp cloth, and then place it in a dry and ventilated place. Sprinkle some water on the sand between the two cans frequently. , this can play a role in preservation. The purpose of frequently sprinkling some water between the two jars is to ensure continuous evaporation of water, and evaporation absorbs heat and has a cooling effect; placing this jar in a dry and ventilated place is to speed up the evaporation of water in the sand.
9. After seeing the performance of taking an iron ball from a boiling oil pan, classmate Xiao Li learned that the "oil" in the pan is a mixed liquid composed of oil and vinegar. The boiling point of oil is 287 ℃ and the boiling point of vinegar is only 60 ℃, when the temperature reaches 60℃, the liquid will boil. If it continues to be heated, the temperature of the liquid will not (optional "yes" or "no") rise. The temperature of the iron ball cannot exceed 60℃ during the performance. Only when When all the vinegar has vaporized, continue heating and the liquid temperature will rise.
10. The picture shows several pictures of Xiaoyang swimming in an outdoor swimming pool. The students raised some of the following questions based on the scenes in the picture. Among them, the ones that meet the scientific questions that can be explored are (D)
A. Can regular swimming achieve the purpose of physical exercise?
B. Is the speed of liquid evaporation related to the temperature of the liquid?
C. Is the speed of liquid evaporation related to the surface area of the liquid?
D. Why did Xiaoyang feel cold after swimming ashore?
Vaporization and liquefaction PPT, Part 3: Expanding exploration and breakthroughs
14. Li Hua dropped one drop of water of the same mass on each of four identical glass plates, and conducted an experimental study as shown in the figure. He found that the speed of water evaporation is related to the temperature of the water, the surface area of the water, and the speed of air flow above the water surface.
(1) By comparing the two pictures A and D, it can be concluded that the speed of water evaporation is related to the speed of air flow above the water surface;
(2) By comparing the two pictures A and C, it can be concluded that the speed of water evaporation is related to the temperature of the water;
(3) In the same natural environment, classmate Li Hua dropped water and alcohol of the same quality on two identical glass plates (as shown in Figures A and B), and controlled their surface areas to be the same. It was found that the The alcohol in B evaporates first, so it can be concluded that when other conditions are the same, the speed of liquid evaporation is also related to the type of liquid;
(4) We know that liquid absorbs heat when it evaporates. Please give an example of applying evaporation to absorb heat: blowing a fan in summer makes you feel cool (just reasonable).
Keywords: PPT courseware for eighth-grade physics volume 1 of the People's Education Press is free to download, vaporization and liquefaction PPT download, changes in the state of matter PPT download, vaporization PPT download, .PPT format;
For more information about the PPT courseware "Changes in the State of Matter: Vaporization and Liquification and Vaporization", please click on the "Changes in the State of Matter ppt Vaporization and Liquification ppt Vaporization ppt" tab.
"Vaporization and Liquefaction" PPT courseware on changes in states of matter (Lesson 2: Liquefaction):
"Vaporization and Liquefaction" PPT courseware on changes in the state of matter (Lesson 2: Liquefaction) Part One: Knowledge Points Basic Knowledge Points Liquefaction 1. In winter, students who wear glasses always have a problem when eating noodles, that is, looking at them while eating We no longer see each other. This is because of the water vapor in the noodles.
"Vaporization and Liquefaction" PPT download of changes in the state of matter:
"Vaporization and Liquefaction" PPT Download of Changes in State of Matter Part One: New Knowledge Summary 1. Vaporization and Liquefaction 1. Vaporization: The process of matter changing from _______ state to _______ state. 2. Liquefaction: The process of matter changing from _______ state to _______ state. 2. Two types of vaporization..
"Vaporization and Liquefaction" PPT on Changes in State of Matter (Liquefaction in Lesson 2):
"Vaporization and Liquefaction" PPT on Changes in State of Matter (Liquefaction in Lesson 2) Part One: Preview Perception 1. Liquefaction 1. Concept. The process by which matter changes from ________ to ________. 2. Method. (1)All gases can liquefy when ________ falls low enough. ..