People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Hunan Education Edition Ninth Grade Chemistry Volume 1
Cantonese Education Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 2
People's Education Press High School Chemistry Compulsory Course 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| People's Education Press High School Chemistry Compulsory Course I | pptx | 6 MB |
Description
"Amount of Matter" Important elements in seawater - sodium and chlorine PPT (Lesson 2 Molar Volume of Gas)
Part One: Learning Objectives
Curriculum Standards
1. Understand the meaning and application of gas molar volume, and appreciate the important role of quantitative research in chemical science.
2. Be able to understand the composition of matter and its chemical changes based on the amount of matter, and use the relationship between the amount of matter, molar mass, and gas molar volume to perform simple calculations.
core competencies
1. Concept of change: Understand the important role of gas molar volume in quantitative chemical research.
2. Macroscopic identification and microscopic analysis: Establish the concept of gas molar volume, and quantitatively understand the composition of matter and the chemical changes of matter based on gas molar volume.
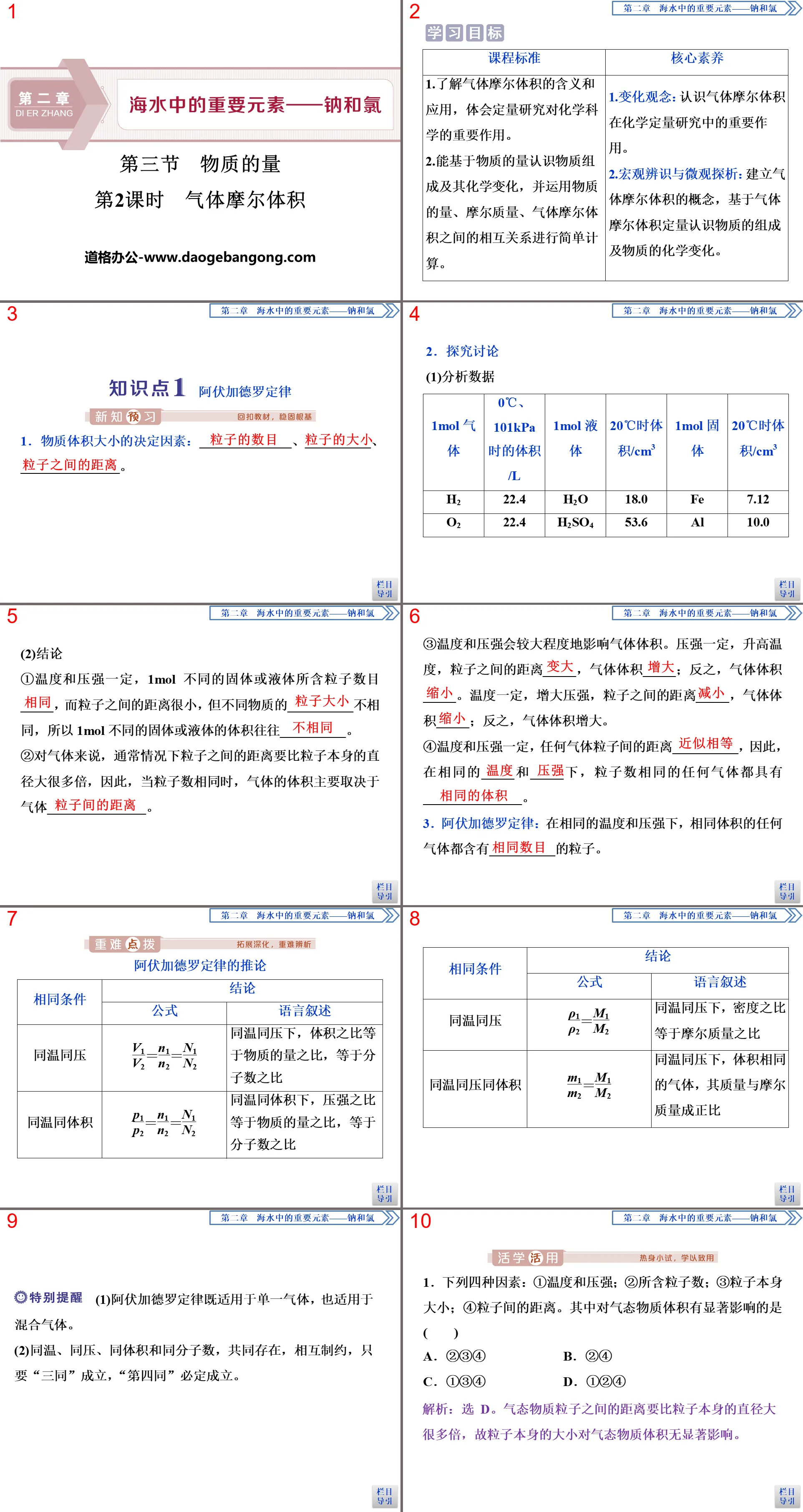
Amount of matter PPT, part 2 content: Knowledge point 1 Avogadro’s law
1. Determinants of the size of a substance: ______________, __________, _______________.
2. Explore and discuss
(1)Analyze data
(2)Conclusion
①The temperature and pressure are constant, the number of particles contained in 1 mol of different solids or liquids is _____, and the distance between the particles is very small, but the __________ of different substances are different, so the volume of 1 mol of different solids or liquids is often __________ .
②For gases, the distance between particles is usually many times larger than the diameter of the particles themselves. Therefore, when the number of particles is the same, the volume of the gas mainly depends on the gas_______________.
③Temperature and pressure will affect the gas volume to a great extent. When the pressure is constant and the temperature increases, the distance between particles _____, and the gas volume _____; otherwise, the gas volume _____. When the temperature is constant and the pressure increases, the distance between particles _____, and the gas volume _____; conversely, the gas volume increases.
④When the temperature and pressure are constant, the distance between any gas particles is __________. Therefore, under the same _____ and _____, any gas with the same number of particles has _______________.
3. Avogadro's Law: At the same temperature and pressure, the same volume of any gas contains __________ particles.
Troubleshooting
Corollary to Avogadro's law
special reminder
(1) Avogadro’s law applies to both single gases and mixed gases.
(2) Same temperature, same pressure, same volume and same number of molecules coexist and restrict each other. As long as the "three samenesses" are established, the "fourth sameness" must be true.
use as you learn
1. The following four factors: ① temperature and pressure; ② the number of particles contained; ③ the size of the particles themselves; ④ the distance between particles. Among them, the ones that have a significant impact on the volume of gaseous substances are ()
A. ②③④ B. ②④
C. ①③④ D. ①②④
2. At present, 1molH2 and 1molO2 are at the same temperature and pressure. Which of the following statements is correct ()
A. Same mass, different volumes
B. Same number of molecules but different masses
C. Same volume but different number of molecules
D. Same volume, different number of atoms
3. At a certain temperature and pressure, 2 volumes of X2 gas and 3 volumes of Y2 gas react completely to produce 2 volumes of gas compound Z, then the chemical formula of Z may be ()
A. XY3B. XY C. X3YD. X2Y3
Amount of substance PPT, part 3 content: Knowledge point 2 Gas molar volume
1. Definition: The volume occupied by gas in unit ____________.
2. Units and symbols: The units are ______ and _______, and the symbol is Vm.
3. Expression: Vm=Vn.
4. Special case: Under standard conditions (i.e. 0°C and 101kPa), the molar volume of gas is approximately ____________.
5. Four Notes on Using Gas Molar Volume
Troubleshooting
Gas molar volume related calculations
use as you learn
1. Which of the following descriptions about the molar volume of gases is correct ()
A. The volume occupied by a gas per unit amount of substance is the gas molar volume
B. The molar volume of gas at normal temperature and pressure is approximately 22.4L
C. The molar volume of gas under standard conditions is approximately 22.4L
D. The molar volumes of gases with the same amount of substance are also the same
2. It is known that the mass of 5.6L of X gas molecules under standard conditions is 8g, then the molar mass of X gas is ()
A. 16 GB. 32g
C. 64g/mol D. 32g/mol
3. (2019•Zhengzhou Senior High School Test) Use NA to represent the value of Avogadro's constant. The correct one in the following description is ()
A. At normal temperature and pressure, the number of atoms contained in 11.2LCO2 is 1.5NA
B. At normal temperature and pressure, the number of oxygen atoms contained in 48gO3 is 3NA
C. Under standard conditions, the number of molecules contained in 22.4LH2O is NA
D. Under standard conditions, the number of atoms contained in 22.4LH2 is NA
Quantity of matter PPT, part 4 content: Qualification examination training
1. Under the same temperature and pressure, if two gases occupy different volumes, the main reason is ()
A. Gas molecules vary in size
B. The average distance between gas molecules is different
C. The amount of gas substances varies
D. Gases have different molar masses
2. When the volume ratio of methane to oxygen in the gas is 1:2, it is extremely explosive. At this time, the mass ratio of methane to oxygen is ()
A. 1:1 B. 1:2
C. 1:3 D. 1:4
3. The volume of the following substances is approximately 22.4L ()
A. 1molH2O under standard conditions
B. 36.5gHCl at 20℃, 101kPa
C. 17gNH3 at normal temperature and pressure
D. Mixed gas of 0.4molH2 and 0.6molO2 at 0℃ and 101kPa
Keywords: Free download of the PPT courseware for high school chemistry compulsory course 1 of the People's Education Press, PPT download of the amount of matter, PPT download of the important elements sodium and chlorine in sea water, PPT download of gas molar volume, .PPT format;
For more information about "The Amount of Important Elements Sodium and Chlorine Substances in Seawater by Gas Molar Volume" PPT courseware, please click on the PPT tag of "The Amount of Important Elements Sodium and Chlorine PPT Substances in Seawater by Gas Molar Volume".
"Physical Quantities Commonly Used in Chemistry - Quantity of Substances" Understanding Chemical Science PPT Courseware (Lesson 3: Amount and Concentration of Substances):
"Amount of Physical Quantities Commonly Used in Chemistry" Understanding Chemical Science PPT Courseware (Lesson 3: Amount and Concentration of Substances) Part One Content: Learning Objectives Course Standards 1. Use the amount of substances, molar mass, molar volume of gases, and the amount and concentration of substances The interrelationship between...
"Commonly used physical quantities in chemistry - the amount of matter" PPT courseware for understanding chemical science (lesson 2: molar volume of gases):
"Amount of Physical Quantities Commonly Used in Chemistry" Understanding Chemical Science PPT Courseware (Lesson 2: Gas Molar Volume) Part One Content: Learning Objectives Course Standards Use the relationship between the amount of matter, molar mass, and gas molar volume to perform simple calculations . nuclear..
"Commonly used physical quantities in chemistry - the amount of matter" PPT courseware for understanding chemical science (lesson 1, the amount of matter and its unit molar mass):
"Amount of Physical Quantities Commonly Used in Chemistry" Understanding Chemical Science PPT Courseware (Lesson 1 Amount of Substance and Its Unit Molar Mass) Part One Content: Learning Objectives Course Standards Can understand the composition of matter and its chemical changes based on the amount of matter, and use The amount of matter,...
File Info
Update Time: 2024-11-03
This template belongs to Chemistry courseware People's Education Press High School Chemistry Compulsory Course I industry PPT template
"Amount of Matter" Important elements in seawater - sodium and chlorine PPT (Lesson 2: Gas molar volume) Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Amount of Matter" Important elements in seawater - sodium and chlorine PPT (Lesson 2: Gas molar volume) is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Amount of Matter" Important elements in seawater - sodium and chlorine PPT (Lesson 2: Gas molar volume), due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview