"Acidic Solutions and Alkaline Solutions" Initial Acids, Bases and Salts PPT Courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Acidic Solutions and Al... | 24625次 | 0.00 | Free Download |
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Acidic Solutions and Alkaline Solutions" Initial Acids, Bases and Salts PPT Courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Acidic Solutions and Alkaline Solutions" Initial Acids, Bases and Salts PPT Courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)

Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see








Authoritative PPT Summary
"Acidic Solutions and Alkaline Solutions" Initial Acids, Bases and Salts PPT Courseware
Simple classification of substances:
substance
Mixture A substance composed of two or more substances (such as air, solution, etc.)
Pure substance
A substance composed of a substance. (such as oxygen, carbon dioxide, potassium permanganate, etc.)
Elemental metal element (such as iron, magnesium, etc.)
Non-metallic elements (such as carbon, oxygen, etc.)
Pure substances composed of the same elements
compound oxide
Composed of two elements, one of which is a compound of oxygen
Other compounds (acid and alkali salts, etc.)
1. Acidic solution and alkaline solution
In daily life, what substances have left a "sour impression" on you?
life inspiration
The wine tastes spicy - there is ethanol in the wine
Sugar water has sweetness - there is sucrose in sugar water
Salt water has a salty taste - Salt water has sodium chloride
Soda water has a bitter taste - it contains alkaline substances
Vinegar and yogurt have a sour taste - they contain acidic substances
Normally, you are not allowed to taste the taste of chemical reagents, so how do we determine whether a substance is acidic or alkaline?
Recall: What do you see when you pass carbon dioxide into purple litmus solution?
CO2+H2O==H2CO3
Can solutions of vinegar, vermicelli, and other sour substances turn purple litmus test solution red?
2. The acidity and alkalinity of the solution
Litmus or phenolphthalein test solution can be used to test the acidity and alkalinity of a certain solution, but can the acidity and alkalinity of two solutions be compared? For example: vinegar and dilute hydrochloric acid are both acidic. Which one is more acidic?
Vermicelli juice, white vinegar, and dilute hydrochloric acid have different acidic strengths, and lime water, ammonia water, and soapy water have different alkaline strengths. Therefore, people use pH to express the degree of acidity or alkalinity of a solution.
Notice
The smaller the pH value of an acidic solution, the stronger the acidity; conversely, the larger the pH value, the weaker the acidity (inverse ratio).
The larger the pH value of an alkaline solution, the stronger the alkalinity, and conversely, the smaller the pH value, the weaker the alkalinity (proportional).
Observe the picture below and fill in the blanks below:
1. The acidity and alkalinity of the solution: pH=7, the solution is ____; pH<7, the solution is ____; pH>7, the solution is ____.
2. The relationship between the pH of the solution and the acidity and alkalinity of the solution: the greater the pH, ________; the smaller the pH, ________.
3. The relationship between the acidity and alkalinity of the solution and life activities
Reading materials:
The pH of body fluids in healthy people must be maintained within a certain range. If the pH of body fluids exceeds the normal range, it will lead to physiological dysfunction or disease, or even "acidosis." If there is too much gastric acid secretion, the pH of the gastric juice drops below the normal level, and symptoms such as stomach pain may easily occur.
Reading materials:
Most crops are suitable for growing in soil that is close to neutral (pH between 6.5 and 7.5). Soil that is too acidic (pH less than 4) or too alkaline (pH greater than 8) is not suitable for the growth of crops.
When a certain mass fraction of an acidic or alkaline solution is diluted with water, the relationship between the pH of the solution and the volume (V) of the added water is:
For alkaline solutions, after adding water, the alkalinity of the solution weakens and the pH decreases, gradually approaching 7, but it will never equal 7.
For acidic solutions, after adding water, the acidity of the solution weakens and the pH increases, gradually approaching 7, but it will never equal 7.
Can you come up with the answer?
1. Commonly used acid-base indicators in laboratories include ( ) test solution and ( ) test solution, among which ( ) can change color when exposed to acid or alkali.
2. Acid-base indicators are commonly used in laboratories to test the solution ( ), and pH is commonly used to measure the solution ( ).
3. Of the following liquids in the normal human body, the one that is acidic and the most acidic is ().
A.Plasma B.Pancreatic juice C.Gastric juice D.Saliva
4. Benzoic acid can be used as a food preservative, and its acidity is stronger than acetic acid. Which of the following assumptions about the properties of benzoic acid is unreasonable ( ).
A. The pH of the benzoic acid solution is greater than 7. B. The pH of the benzoic acid solution is less than 7.
C. Benzoic acid can turn purple litmus test solution red
D. Benzoic acid cannot change color of colorless phenolphthalein test solution
Improvement exercises:
1. The following pH value indicates the most acidic solution:
A, Ph=0 B, pH =1
C, pH =7 D, pH =14
2. Doctors remind patients with hyperacidity to eat less apples. Then the pH of apples:
A, greater than 7 B, less than 7
C. Equal to 7 D. Unable to judge
3. When sodium hydroxide solution is added dropwise to dilute hydrochloric acid, the correct pH change of the solution is:
pH and human health
The pH value of human body fluids is normal when it is 7.35~7.45;
When the pH value of human body fluids is <7.35, it is in a sub-healthy state;
When the pH value of human body fluids = 6.9, it becomes a vegetative state;
Death occurs when the pH value of human body fluids is 6.85~6.45.
Simple classification of acid-reducing foods
Acidic foods: animal foods other than milk
Alkaline foods: plant foods other than whole grains
Neutral food: oil, salt, coffee, tea, etc.
Acid-base classification of common foods
Strong acidity - egg yolks, cheese, pastries made with white sugar, persimmons, dried bonito, etc.
Medium acidity - ham, bacon, chicken, pork, eel, beef, bread, wheat, butter, etc.
Weakly acidic - rice, peanuts, beer, seaweed, loach, etc.
Weakly alkaline - red beans, radishes, apples, cabbage, onions, tofu, etc.
Medium alkaline - soybeans, carrots, tomatoes, bananas, oranges, pumpkins, strawberries, egg whites, prunes, lemons, spinach, etc.
Strongly alkaline - grapes, wine, kelp sprouts, kelp, etc. Especially for natural green algae, the best time to drink is from 9 a.m. to 11 a.m.
common acids common bases
Nitric acid HNO3 Sodium hydroxide NaOH
Sulfate H2SO4 Calcium hydroxide Ca(OH)2
Hydrochloric acid HCl Ammonia NH3·H2O
Carbonic acid H2CO3
Common acidic salt solutions Common alkaline salt solutions
Ammonium chloride NH4Cl Sodium carbonate Na2CO3
Copper sulfate CuSO4 Sodium bicarbonate NaHCO3
Ferric chloride FeCl3
Keywords: Initial acids, bases and salts teaching courseware, acidic solutions and alkaline solutions teaching courseware, Hunan Education Edition 9th grade chemistry PPT courseware download, second volume, 9th grade chemistry slide courseware download, initial acids, bases and salts PPT courseware download, acidity Solution and alkaline solution PPT courseware download, .PPT format;
For more information about the PPT courseware "Initial Acids, Bases and Hydrochloric Acid Solutions and Alkaline Solutions", please click on the Initial Acids, Bases and Salts ppt Acidic Solutions and Alkaline Solutions ppt tag.
"Acidic Solutions and Alkaline Solutions" Initial Acids, Bases and Salts PPT Courseware 3:
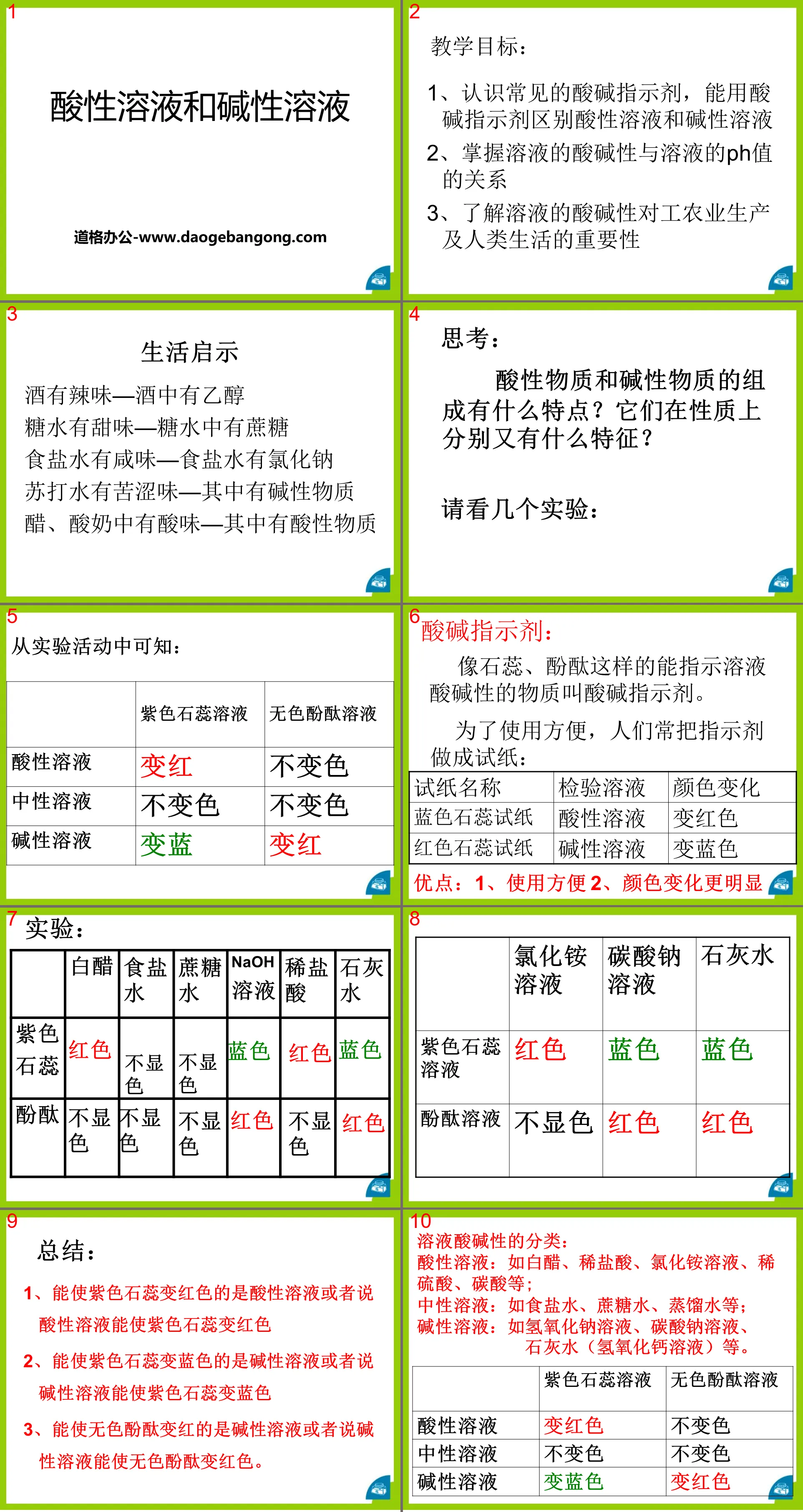
"Acidic Solution and Alkaline Solution" Initial Acid, Alkali and Salt PPT Courseware 3 Teaching Objectives: 1. Recognize common acid-base indicators and be able to use acid-base indicators to distinguish acidic solutions and alkaline solutions 2. Master the acid-base of solutions The relationship between the property and the pH value of the solution 3. Understand the solution...
"Acidic Solutions and Alkaline Solutions" Initial Acids, Bases and Salts PPT Courseware 2:
"Acidic Solutions and Alkaline Solutions" Initial Acids, Alkalis and Salts PPT Courseware 2 [Association and Inspiration] In daily life, we often feel the fact that: tea and soda water have a bitter taste; vinegar , Yogurt has a sour taste; sugar water has a sweet taste; wine has a spicy taste, etc. Different foods are...