| Category | Format | Size |
|---|---|---|
| Hunan Education Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"Law of Conservation of Mass" Chemical Changes and Their Representation PPT Courseware
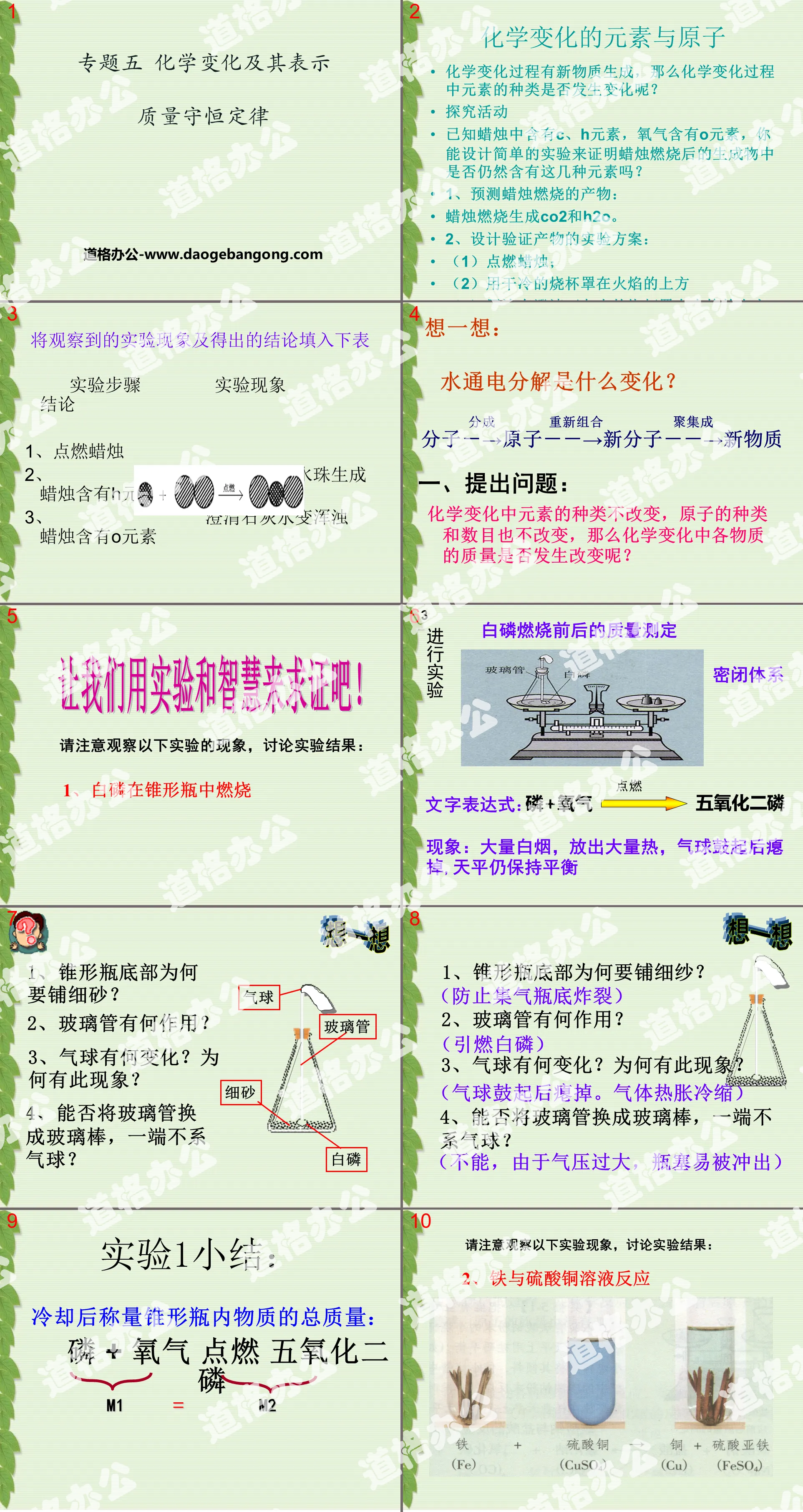
Elements and atoms of chemical changes
New substances are generated during chemical changes, so do the types of elements change during chemical changes?
Inquiry activities
It is known that candles contain C and H elements, and oxygen contains O elements. Can you design a simple experiment to prove whether the products after burning candles still contain these elements?
1. Predict the products of candle burning:
Candles burn to produce CO2 and H2O.
2. Design the experimental plan for product verification:
(1) Light the candle;
(2) Cover the flame with a dry and cold beaker
(3) Cover the flame with a beaker coated with clarified lime water
Think about it:
What happens when water is decomposed by electricity?
Molecule-→Atom--→New Molecule--→New Substance
1. Ask questions:
The types of elements do not change during chemical changes, and the types and numbers of atoms do not change. So does the mass of each substance change during chemical changes?
Mass determination of white phosphorus before and after combustion
Literal expression: phosphorus + oxygen → phosphorus pentoxide
Phenomenon: A large amount of white smoke and a large amount of heat are emitted. The balloon bulges and then deflates, but the scale remains balanced.
think about it
1. Why is the bottom of the Erlenmeyer flask covered with fine sand?
2. What is the function of glass tube?
3. What happens to the balloon? Why does this happen?
4. Can the glass tube be replaced with a glass rod without a balloon attached to one end?
Key points of the concept of the law of conservation of mass:
The sum of the masses of each substance participating in a chemical reaction is equal to the sum of the masses of each substance produced after the reaction.
1. "Sum" means counting the reactants and products in various states. Gases such as precipitation and invisible to the naked eye should also be considered.
2. The sum of the masses of each substance participating in a chemical reaction is not the sum of the arbitrary masses of each substance, and the mass of substances not participating in the reaction cannot be calculated.
3. The "conservation" of the law of conservation of mass refers to the conservation of mass, not the conservation of volume or the number of molecules of reactants.
What is the scope of application of the law of conservation of mass? What use does learning this law have for us?
The law of conservation of mass applies to all chemical reactions.
applicable laws
1. Make relevant calculations
2. Speculate the composition of some substances
3. Explain some experimental facts
A simple application of the law of conservation of mass.
1. When 6g of carbon is burned in sufficient oxygen to produce 22g of carbon dioxide, ____g of oxygen participates in the reaction.
2. Heat a mixture of 25 g potassium chlorate and 1 g manganese dioxide until it is completely decomposed and the residual solid mass is 14 g, then ____g of oxygen is produced.
3. It is known that paraffin is the main component of candles. The products of complete combustion of candles in the air are CO2 and H2O. It is judged that paraffin must contain _______ elements and may contain ___ elements.
4. In the chemical reaction 2XY2+Y2=2Z, the chemical formula of Z is ( )
A.X2Y3 B.XY3
C、X2Y6 D、X2Y4
Keywords: Chemical Changes and Their Representation Teaching Courseware, Law of Conservation of Mass Teaching Courseware, Hunan Education Edition Ninth Grade Chemistry PPT Courseware Download, Ninth Grade Chemistry Slide Courseware Download, Chemical Change and Its Representation PPT Courseware Download, Law of Conservation of Mass PPT Courseware Download, .PPT format;
For more information about the "Law of Conservation of Mass Chemical Changes and Their Representations" PPT courseware, please click on the PPT Label of "Law of Conservation of Mass Chemical Changes and Their Representations" PPT.
"Law of Conservation of Mass" Chemical Equations PPT Courseware (Lesson 2):
"Law of Conservation of Mass" Chemical Equations PPT Courseware (Lesson 2), 12 pages in total. Introduction of new lesson: Which one is better to express chemical reactions in various ways? Type 3 is the best if it requires simplicity and versatility. Like formula ③, a chemical formula is used to express a chemical reaction,...
"Law of Conservation of Mass" Chemical Equations PPT Courseware (Lesson 1):
"Law of Conservation of Mass" Chemical Equations PPT Courseware (Lesson 1), 15 pages in total. Introduction to New Lesson We already know that the result of a chemical reaction is the conversion of reactants into products. So, how do the masses of reactants and products change during a chemical reaction?
"Law of Conservation of Mass" Chemical Equation PPT (Lesson 2):
"Law of Conservation of Mass" Chemical Equations PPT (Lesson 2), 16 pages in total. Learning Objectives 1. Through the chemical equation of carbon combustion, be able to obtain and state the relevant reaction information from the specific chemical equation, and summarize the meaning of the chemical equation. 2. Through carbon..
File Info
Update Time: 2024-07-06
This template belongs to Chemistry courseware Hunan Education Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Law of Conservation of Mass" Chemical Changes and Their Representation PPT Courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Law of Conservation of Mass" Chemical Changes and Their Representation PPT Courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Law of Conservation of Mass" Chemical Changes and Their Representation PPT Courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview