People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Hunan Education Edition Ninth Grade Chemistry Volume 1
Cantonese Education Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 2
People's Education Press High School Chemistry Compulsory Course 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Hunan Education Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"Multicomponent Air" Air and Water PPT Courseware 3
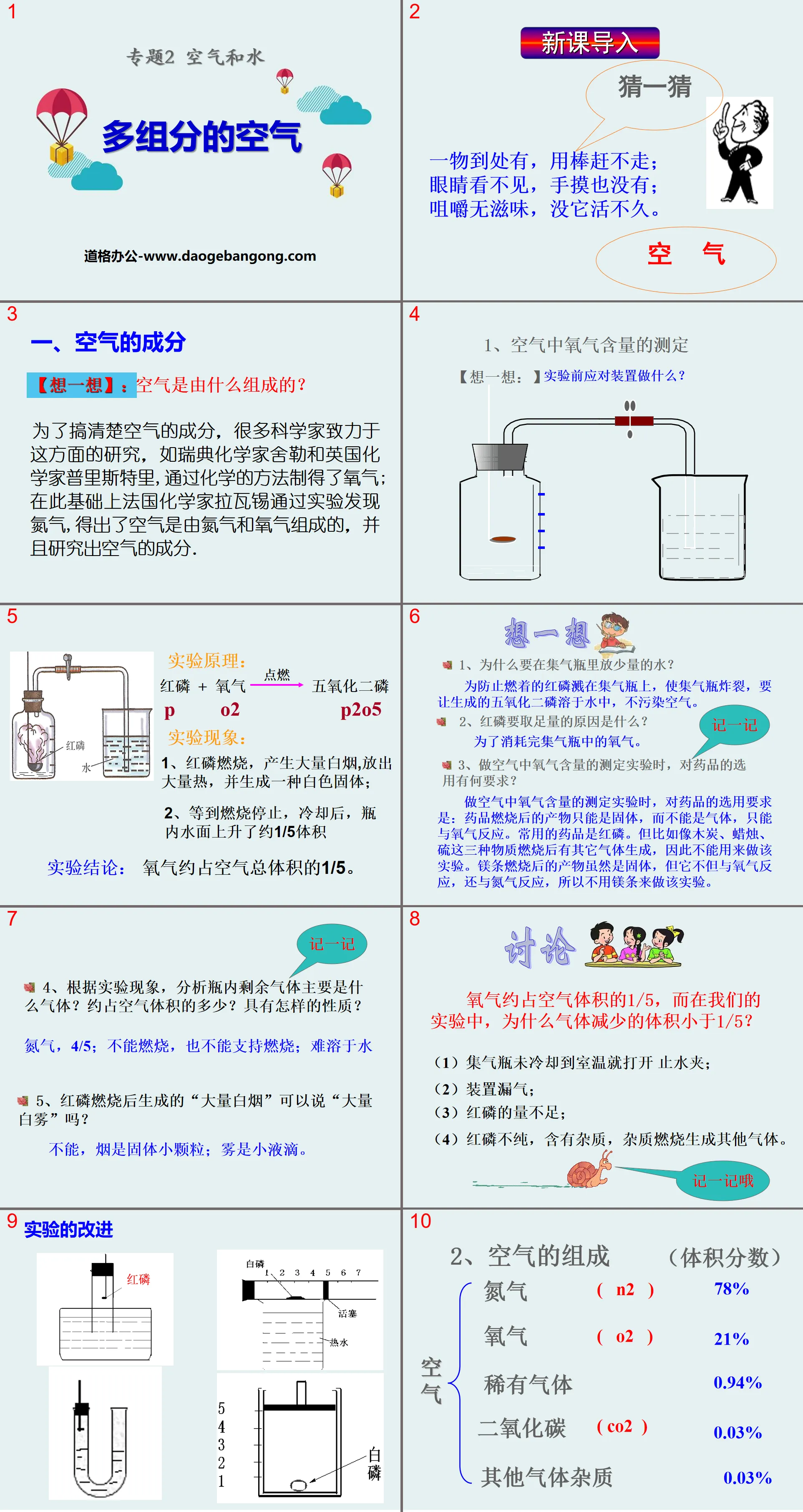
1. Composition of air
[Think about it]: What is air made of?
In order to understand the composition of air, many scientists have devoted themselves to research in this area. For example, the Swedish chemist Scheler and the British chemist Priestley produced oxygen through chemical methods; on this basis, the French chemist Lavoisier passed The experiment discovered nitrogen, concluded that air is composed of nitrogen and oxygen, and studied the composition of air.
1. Determination of oxygen content in air
[Think about it:] What should I do with the device before the experiment?
Experimental principle:
Red phosphorus + oxygen → phosphorus pentoxide
Experimental phenomena:
1. Red phosphorus burns, producing a large amount of white smoke, releasing a large amount of heat, and producing a white solid;
2. Wait until the burning stops and after cooling, the water level in the bottle will rise by about 1/5 of the volume.
Experimental conclusion: Oxygen accounts for about 1/5 of the total volume of air.
think about it
1. Why should we put a small amount of water in the gas collecting bottle?
In order to prevent the burning red phosphorus from splashing on the gas collecting bottle and causing the gas collecting bottle to explode, the generated phosphorus pentoxide should be dissolved in the water so as not to pollute the air.
2. What is the reason for taking enough red phosphorus?
In order to consume all the oxygen in the gas cylinder.
3. When conducting an experiment to measure the oxygen content in the air, what are the requirements for the selection of drugs?
When doing an experiment to measure the oxygen content in the air, the requirements for the selection of drugs are: the products after combustion of the drugs can only be solids, not gases, and can only react with oxygen. A commonly used drug is red phosphorus. However, for example, three substances such as charcoal, candles, and sulfur produce other gases after burning, so they cannot be used for this experiment. Although the product after burning a magnesium bar is solid, it reacts not only with oxygen but also with nitrogen, so a magnesium bar is not used for this experiment.
4. According to the experimental phenomenon, what is the main gas remaining in the analysis bottle? Approximately how much volume of air does it occupy? What properties does it have?
Nitrogen, 4/5; cannot burn or support combustion; poorly soluble in water
5. Can the "large amount of white smoke" generated after the combustion of red phosphorus be called "large amount of white mist"?
No, smoke is small solid particles; fog is small droplets.
discuss
Oxygen accounts for about 1/5 of the volume of air, and in our experiment, why did the gas reduce its volume by less than 1/5?
(1) Open the water stopper before the gas cylinder cools to room temperature;
(2) The device is leaking;
(3) The amount of red phosphorus is insufficient;
(4) Red phosphorus is impure and contains impurities, which burn to generate other gases.
2. Properties and uses of nitrogen
physical properties
Colorless, odorless gas. Insoluble in water and low melting point. Density: 1.251g/L
chemical properties
Neither can burn nor support burning
3. Properties and uses of rare gases
[Think about it]: What are the properties and uses of rare gases? (P30-32)
(1) Rare gases are the general name for gases such as helium, neon, argon, krypton, xenon, and radon.
(2) Rare gases are colorless, odorless, and very inactive chemically.
(3) Purpose: protective gas, electric light source laser technology, low temperature anesthesia
Thinking: What is the difference between air and oxygen, mineral water and distilled water?
In addition to oxygen, air also contains nitrogen, rare gases and other substances, while oxygen has only one substance; mineral water has many minerals besides water, while distilled water only has one substance: water.
A substance like air that is a mixture of two or more substances is called a mixture.
Nitrogen, oxygen, carbon dioxide, etc. are each composed of one substance, and they are all pure substances.
4. Pure substances and mixtures
Mixture: A mixture of two or more substances
Such as: air, sea water, river water, mineral water, etc.
Pure substance: composed of a substance
Such as: N2, O2, CO2, P, P2O5, H2O
Pure substances can be represented by special chemical symbols. For example, nitrogen, oxygen, and carbon dioxide can be represented as N2, O2, CO2, etc. respectively.
Class exercises
1. Which of the following substances are pure substances ( )
A A rare gas separated from the air
Part B frozen distilled water
C Salt water D 75% medicinal alcohol
2. Among the following groups of substances, the former is a pure substance and the latter is a mixture ( )
A. Carbon dioxide Distilled water B. iron ore salt water
C. Hydrogen CaCO3 D. nitrogen air
3. Which of the following water is not a mixture ( )
A mineral water B tap water C river water D ice-floating water
4. Calculated by volume, the most abundant gas in the air is ( )
A oxygen B nitrogen
C carbon dioxide D rare gas
5. The volume of oxygen in 100 liters of air is approximately ( )
A 78 liters B 78% C 21 liters D 21%
6. The volume ratio of oxygen to nitrogen in the air is approximately ( )
A 4:1 B 1:4 C 1:5 D 5:1
Keywords: air and water teaching courseware, multi-component air teaching courseware, Hunan Education Edition ninth-grade chemistry PPT courseware download, ninth-grade chemistry slide courseware download, air and water PPT courseware download, multi-component air PPT courseware Download, .PPT format;
For more information about the "Air and Water Multi-Component Air" PPT courseware, please click the Air and Water ppt Multi-Component Air ppt tag.
"Multicomponent Air" Air and Water PPT Courseware 2:
"Multicomponent Air" Air and Water PPT Courseware 2 Thoughts 1. If there was no air in the world we live in, what would our world look like? 2. The air is all around you. Can you describe its physical properties? 3. Air is a single...
"Multicomponent Air" Air and Water PPT Courseware:
"Multicomponent Air" Air and Water PPT Courseware Learning Objectives Determination of Oxygen Content in the Air Experiment 1. Red phosphorus combustion experimental phenomenon, text expression, conclusion 2. Reasons for small experimental results 3. Red phosphorus is the key to the success of the experiment The phenomenon of burning in air? ..
File Info
Update Time: 2024-10-07
This template belongs to Chemistry courseware Hunan Education Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Multicomponent Air" Air and Water PPT Courseware 3 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Multicomponent Air" Air and Water PPT Courseware 3 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Multicomponent Air" Air and Water PPT Courseware 3, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview