| Category | Format | Size |
|---|---|---|
| Zhejiang Education Edition Eighth Grade Science Volume 1 | pptx | 6 MB |
Description
"Dissolution of Substances in Water" PPT
Part One: Review:
1. Solute: the substance that is dissolved
Solvent: A substance that dissolves other substances
Solution: A substance obtained by dissolving a solute in a solvent
Solutions that usually do not specify a solvent generally refer to aqueous solutions.
2. Solution: It is a uniform, stable and transparent mixture.
Suspension: A mixture of small solid particles suspended in a liquid.
Emulsion: A mixture of small droplets dispersed in an (immiscible) liquid.
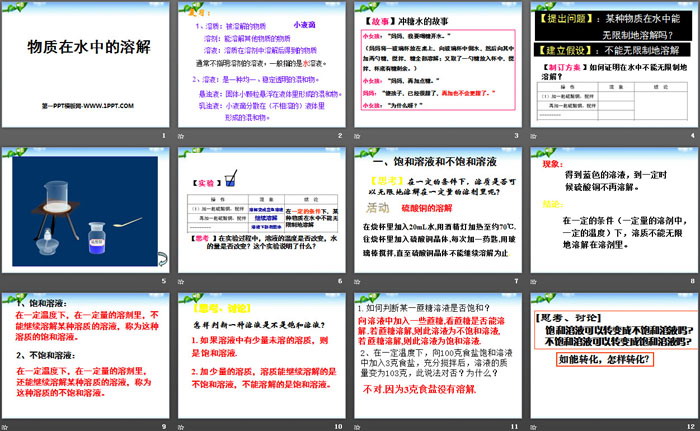
Dissolution of substances in water PPT, Part 2: Saturated solutions and unsaturated solutions
[Thinking] Under certain conditions, can a solute be dissolved infinitely in a certain amount of solvent?
Dissolution of copper sulfate
Add 20mL of water to the beaker and heat it to about 70°C with an alcohol lamp. Add copper sulfate crystals to the beaker, one spoon at a time, and stir with a glass rod until the copper sulfate crystals can no longer dissolve.
Phenomenon: A blue solution is obtained, and the copper sulfate no longer dissolves after a certain time.
Conclusion: Under certain conditions (a certain amount of solvent, a certain temperature), the solute cannot dissolve infinitely in the solvent.
1. Saturated solution:
A solution that cannot continue to dissolve a certain solute in a certain amount of solvent at a certain temperature is called a saturated solution of this solute.
2. Unsaturated solution:
A solution that can continue to dissolve a certain solute in a certain amount of solvent at a certain temperature is called an unsaturated solution of this solute.
Dissolution of substances in water PPT, part 3 content: [Thinking and discussion]
How to tell if a solution is saturated?
1. If there is a small amount of undissolved solute in the solution, it is a saturated solution.
2. Add a small amount of solute. The solute that can continue to dissolve is an unsaturated solution, and the solute that cannot be dissolved is a saturated solution.
think:
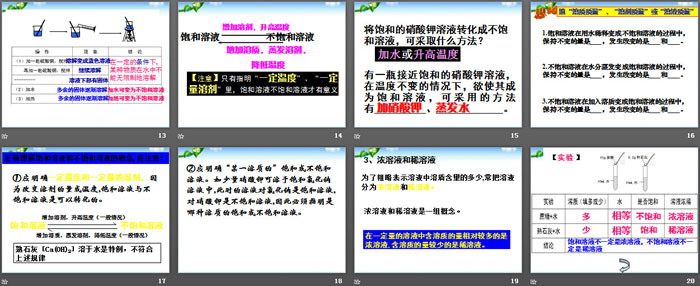
Fill in the "mass of solute", "mass of solvent" or "mass of solution"
1. When a saturated solution is diluted with water to become an unsaturated solution, the quantity that remains unchanged is _____, and the quantities that change are _____ and _____.
2. When the water evaporates from an unsaturated solution to become a saturated solution, the quantity that remains unchanged is _____, and the quantities that change are _____ and _____.
3. When a solute is added to an unsaturated solution to become a saturated solution, the quantity that remains unchanged is _____, and the quantities that change are _____ and _____.
Dissolution of substances in water PPT, Part 4 content: Classroom exercises
1. At a certain temperature, the saturated solution of a certain substance must be ( )
A. Very concentrated solution
B. Very dilute solution
C. A solution in which the solute can continue to dissolve when the solute is added
D. Add the solute and the solute cannot continue to dissolve in the solution
2. Which of the following statements is correct ( )
A. In a certain amount of solvent, a saturated solution of table salt is more concentrated than its unsaturated solution.
B. A concentrated solution must be a saturated solution, and a dilute solution must be an unsaturated solution.
C. A saturated solution of potassium nitrate at 20°C will remain a saturated solution when the temperature rises to 60°C, other conditions remaining unchanged.
D. For solutions of the same solute, at a certain temperature, the saturated solution is more concentrated than the unsaturated solution.
3. Decide whether the following statements are correct:
① A saturated solution of the same substance must be more concentrated than an unsaturated solution
② At a certain temperature, add a small amount of potassium nitrate to the potassium nitrate solution. If the quality of the solution remains unchanged, the solution is a saturated solution.
③ A saturated solution of the same substance must have more solute than an unsaturated solution.
④At a certain temperature, if the mass of the solute does not change, the unsaturated solution cannot be turned into a saturated solution.
⑤ A saturated solution of sodium chloride at a certain temperature must not be able to continue to dissolve sucrose.
Keywords: Zhejiang Education Edition eighth grade science PPT courseware free download, dissolution of substances in water PPT download, .PPT format;
For more information about the "Dissolution of Substances in Water" PPT courseware, please click on the "Dissolution of Substances in Water" PPT tab.
"Solubility of different substances in water" Dissolution PPT download:
"Solubility of Different Substances in Water" Dissolution PPT Download Part One Content: Solubility of Salt and Baking Soda in Water 1. Comparatively observe the solubility of salt and baking soda in water. Experimental purpose: By putting salt and baking soda into water respectively Dissolved in water, than...
"Solubility of different substances in water" Dissolution PPT:
"The Solubility of Different Substances in Water" Dissolution PPT Part 1: The solubility of salt and baking soda in water. Do you know them? Baking soda is in the form of white solid powder and is widely used in industrial and agricultural production. It is also the most widely used in the food industry..
"Solubility of different substances in water" Dissolution PPT courseware 4:
"The Solubility of Different Substances in Water" Dissolution PPT Courseware 4 The solubility of salt and baking soda in water. Guess: Which one has the stronger solubility in water, salt or baking soda? Discussion: How can this experiment be fair? Research Experiment Research Question: Food...
File Info
Update Time: 2024-06-10
This template belongs to science courseware Zhejiang Education Edition Eighth Grade Science Volume 1 industry PPT template
"Dissolution of Substances in Water" PPT Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Dissolution of Substances in Water" PPT is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Dissolution of Substances in Water" PPT, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview