| Category | Format | Size |
|---|---|---|
| People's Education Press High School Chemistry Compulsory Course I | pptx | 6 MB |
Description
"End-of-Chapter Integration Improvement" Periodic Law of Material Structure Elements PPT
Part I: 1. Judgment of the metallicity and non-metallicity of elements
1. Judgment of metallicity strength
(1) Judging from the periodic table of elements
①In the same period, from left to right, the metallicity of elements gradually weakens.
②In the same main group, from top to bottom, the metallicity of elements gradually increases.
(2) Judging based on the order of metal activity
→K Ca Na Mg Al Zn Fe Sn Pb (H) Cu Hg Ag Pt Au The activity of the metal element is weakened, and the metallicity of the element is also weakened
(3) Judgment based on the properties of the elements and their compounds
①The more violent the reaction between metal elements and water or acid, the stronger the metallicity of the element.
②The stronger the alkalinity of the hydrate corresponding to the highest valence oxide, the stronger the metallicity of the element.
(4) Judging from the replacement reaction between metal elements
If Xn++Y�→X+Ym+, then Y is more metallic than X.
(5) Judgment based on the oxidation strength of ions
The stronger the oxidizing property of the metal cation, the weaker the metallicity of the element, e.g.
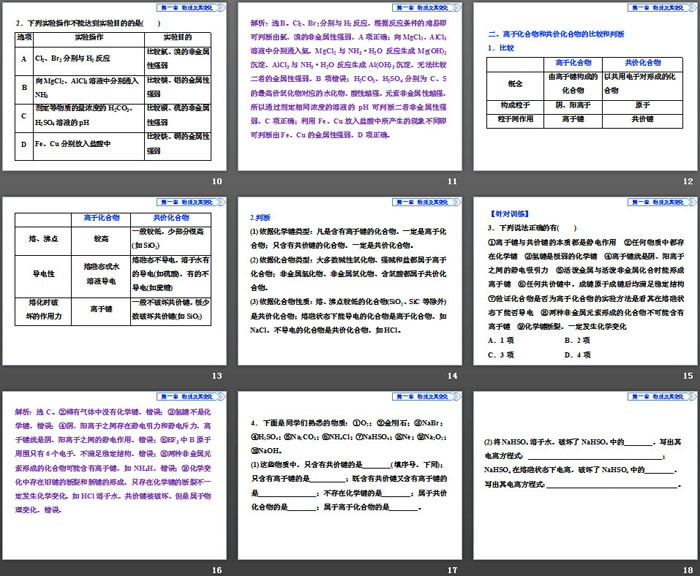
Cu2+>Fe2+, then metallic Cu 2. Judgment of the strength of non-metallic property (1) Judging from the periodic table of elements ①In the same period, from left to right, the non-metallicity of elements gradually increases. ②In the same main group, from top to bottom, the non-metallic properties of elements gradually weaken. (2) Judgment based on the properties of the elements and their compounds ①The easier it is for the element to combine with hydrogen (or the more stable the hydride), the stronger the non-metallic nature of the element. ②The stronger the acidity of the hydrate corresponding to the highest valence oxide, the stronger the non-metallic nature of the element. (3) Judging from the replacement reaction between non-metallic elements If An-+B�→A+Bm-, then B is more non-metallic than A. (4) Judgment based on the reducing ability of ions The stronger the reducing property of the non-metallic anion, the weaker the non-metallic property of the element. For example, reducing Cl- special reminder (1) The fundamental basis for judging the strength of an element’s metallicity and non-metallicity is the difficulty of the element losing or gaining electrons, which has nothing to do with the number of electrons lost or gained. For example, Na loses one electron during the reaction, and Mg loses one electron during the reaction. Lost 2 electrons, but metallic Na>Mg. (2) The fluorine element does not have the highest positive valence, and there is no oxygen-containing acid. Therefore, the most acidic hydrate corresponding to the highest-valent oxide is perchloric acid. (3) The oxidation strength of valence-changing metal ions does not necessarily correspond to the metallicity of the element, such as oxidizing Cu2+ Integrate and improve PPT at the end of the chapter, part 2: 2. Comparison and judgment of ionic compounds and covalent compounds 1. Compare 2. Judgment (1) According to the type of chemical bond: any compound containing ionic bonds must be an ionic compound; a compound containing only covalent bonds must be a covalent compound. (2) Based on the type of compound: most basic oxides, strong bases and salts are ionic compounds; non-metal hydrides, non-metal oxides, and oxyacids are all covalent compounds. (3) Based on the properties of the compounds: Compounds with lower melting and boiling points (except SiO2, SiC, etc.) are covalent compounds; compounds that can conduct electricity in the molten state are ionic compounds, such as NaCl, and compounds that do not conduct electricity are covalent compounds, such as HCl. [For training] 3. Which of the following statements is correct () ① The essence of ionic bonds and covalent bonds are electrostatic interactions ② Chemical bonds exist in any substance ③ Hydrogen bonds are extremely weak chemical bonds ④ Ionic bonds are the electrostatic attraction between anions and cations ⑤ When active metals and active non-metals combine, energy Form an ionic bond ⑥ In any covalent bond, the bonding atoms must meet a stable structure after forming a bond ⑦ The experimental method to verify whether a compound is an ionic compound is to see whether it can conduct electricity in the molten state ⑧ Compounds formed by two non-metallic elements do not May contain ionic bonds ⑨ Chemical bonds are broken and chemical changes must occur A. 1 item B. 2 items C. 3 items D. 4 items 4. The following substances are familiar to students: ①O2; ②Diamond; ③NaBr; ④H2SO4; ⑤Na2CO3; ⑥NH4Cl; ⑦NaHSO4; ⑧Ne; ⑨Na2O2; ⑩NaOH. (1) Among these substances, those that only contain covalent bonds are ________ (fill in the number, the same below); those that contain only ionic bonds are __________; those that contain both covalent bonds and ionic bonds are __________; there are no chemical bonds is ________; is a covalent compound is ________; is an ionic compound is ________. (2) Dissolve NaHSO4 in water, destroy the ________ in NaHSO4, and write its ionization equation: ____________________________; NaHSO4 ionizes in the molten state and destroys the ________ in NaHSO4. Write its ionization equation: ____________________________. Integrate and improve PPT at the end of the chapter, the third part: 3. Comprehensive inference on the relationship between "position, structure, and sex" 1. Diagrammatic thinking method 2. Common "Breakthroughs" (1) The special position of main group elements in the periodic table of elements ① Elements whose group number is equal to the periodic number: H, Be, Al. ② Elements whose group number is equal to twice the period number: C, S. ③ Elements whose group ordinal number is equal to three times the periodic ordinal number: O. ④Short-periodic elements whose period number is equal to twice the group number: Li. ⑤Short-periodic elements whose period number is equal to 3 times the group number: Na. ⑥Short-period elements whose algebraic sum of the highest positive valence and the lowest negative valence is zero: C, Si. ⑦The short-period element whose highest positive price is three times the absolute value of the lowest negative price: S. ⑧Except H, the element with the smallest atomic radius: F. ⑨The elements whose highest price is not equal to the family ordinal number: O, F. (2) The particularity of the properties, existence and uses of main group elements ①The element that forms the most compounds (or the element that is the hardest element in nature or the element with the largest mass fraction of hydrogen in gaseous hydride): C. ②The most abundant element in the air: N. ③The element whose hydride is liquid under normal conditions: O. ④The highest-priced oxide corresponds to the most acidic element in the hydrate: Cl. ⑤The elements contained in the lightest element: H; the elements contained in the lightest metal element: Li. ⑥The element with the highest valence oxide and its hydrate that can react with both strong acids and strong bases: Al. ⑦Elements of short-period elements that can react with water to release gas at room temperature: Li, Na, F. ⑧Common elements that can form allotropes: C, P, O, S. [For training] 5. (2018•National College Entrance Examination Paper III) W, The salt YZW reacts with concentrated hydrochloric acid to produce yellow-green gas. This gas reacts with cold caustic soda solution to obtain a solution containing YZW. Which of the following statements is correct () A. The atomic radius is W B. X's hydride aqueous solution is more acidic than Z's C. Both Y2W2 and ZW2 contain non-polar covalent bonds D. Under standard conditions, the elemental state of W is the same as that of X 6. (2017•National College Entrance Examination Paper II) a, b, c, and d are short-period main group elements with increasing atomic numbers. The total number of electrons outside the nucleus of a atom is the same as the number of electrons in the sub-outer layer of b atom; the period number of c is the same as that of the group. The numbers are the same; d and a are in the same family. Which of the following statements is correct () A. Atomic radius: d>c>b>a B. Among the 4 elements, b has the strongest metallicity C. The hydrate of the oxide of c is a strong base D. The oxidizing property of element d is stronger than that of element a. Keywords: PPT courseware for high school chemistry compulsory course 1 from the People's Education Press is available for free download, end-of-chapter integration and improvement PPT download, structure of matter PPT download, periodic law of elements PPT download, .PPT format; For more information about the PPT courseware "Integrating and Improving the Structure of Matter at the End of the Chapter on the Periodic Law of Elements", please click on the PPT tag of Integrating and Improving the Structure of Matter at the End of the Chapter on the Periodic Law of Elements. "Integration and Improvement at the End of Chapter" Elements and Material World PPT: "End-of-Chapter Integration Improvement" Elements and Material World PPT Part One Contents: 1. Classification methods and their application in life 1. Classification of elements and substances (1) Elements exist in nature in free and combined states. Very active elements can only exist in combined states. like.. "End-of-Chapter Integration Improvement" Understanding Chemical Science PPT: "End-of-Chapter Integration Improvement" Understanding Chemical Science PPT Part One Content: 1. Chemical Terms 1. Element symbol (1) stipulates: Represented by the first capital letter of the Latin name of the element or by appending a lowercase letter. (2) Meaning: ① Macroscopically represents an element; ②.. "Integration Improvement at the End of Chapter" Iron Metal Material PPT: "End-of-Chapter Integration Improvement" Iron Metal Materials PPT Part One Content: Method 1 Common Methods of Chemical Calculation Conservation Method [Typical Example 1] Add 100mL1mol/L to a certain amount of Fe, FeO, Fe2O3 mixture Hydrochloric acid, just enough to completely dissolve the mixture, releases 224..
File Info
Update Time: 2024-07-24
This template belongs to Chemistry courseware People's Education Press High School Chemistry Compulsory Course I industry PPT template
"End-of-Chapter Integration Improvement" Periodic Law of Material Structure Elements PPT Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "End-of-Chapter Integration Improvement" Periodic Law of Material Structure Elements PPT is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"End-of-Chapter Integration Improvement" Periodic Law of Material Structure Elements PPT, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview