People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Hunan Education Edition Ninth Grade Chemistry Volume 1
Cantonese Education Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 2
People's Education Press High School Chemistry Compulsory Course 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| People's Education Press High School Chemistry Compulsory Course I | pptx | 6 MB |
Description
"Oxidant and Reducing Agent" Redox Reaction PPT Courseware
Part 1: Essential knowledge and foundation of literacy
1. Oxidizing agent and reducing agent
1. Meaning and relationship
2. Common oxidizing agents and reducing agents
[Think about it] (1) When non-metallic elements are used as reactants in redox reactions, must they be oxidants? Why?
Tip: Not necessarily. In redox reactions, non-metallic elements may act as oxidants, such as Cl2 and O2, or as reducing agents, such as C and H2.
(2) When a metal element is used as a reactant in a redox reaction, must it be a reducing agent? Why?
Tip: Yes. Metals have no negative valence. When used as reactants in redox reactions, they must lose electrons, increase their valence, and serve as reducing agents.
3. Oxidation products and reduction products
(1) Oxidation products: substances produced by the oxidation of reducing agents.
(2) Reduction products: substances produced by the reduction of oxidants.
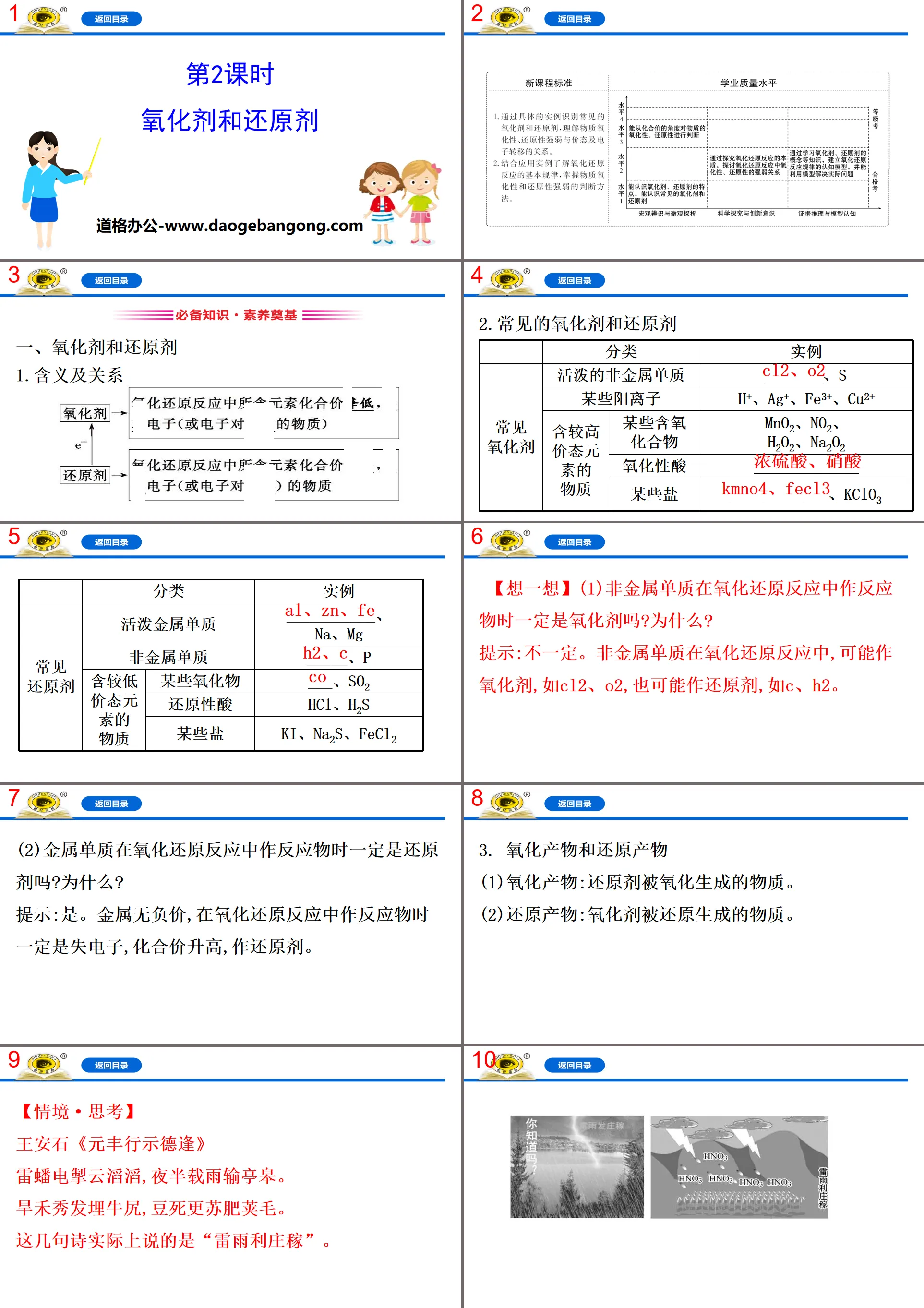
【Situation·Thinking】
Wang Anshi's "Yuanfeng's Journey to Show Virtue"
Thunder and lightning set off the clouds, and rain fell on Tinggao in the middle of the night.
The beautiful hair of the dry grass is buried in the oxen, and the dead beans are replaced by the fat pod hair.
These lines of poetry actually say "Thunderstorms benefit crops."
When lightning and thunder occur, the following reactions occur:
①N2+O2 2NO ②2NO+O2====2NO2
③3NO2+H2O====2HNO3+NO
Analyze whether the three reactions are redox reactions and explain why. It is a redox reaction, indicating the oxidant, reducing agent, oxidation product, and reduction product of the reaction.
Tip: All three reactions are redox reactions. Reaction ①: Oxidant O2, reducing agent N2, NO are both oxidation products and reduction products; Reaction 2: Oxidant O2, reducing agent NO, NO2 are both oxidation products and reduction products; Reaction 3: NO2 is both oxidant and reducing agent, HNO3 is an oxidation product, and NO is a reduction product.
4. Oxidation and reduction
(1) Oxidizing and reducing properties
(2) Relationship between basic concepts:
【Smart Judgment】
(1) Metal cations only have oxidizing properties and do not have reducing properties. ()
Tip:×. Metal cations are in intermediate valence states such as Fe2+, which have both oxidizing and reducing properties.
(2) The more atoms of a metal element lose electrons, the stronger the reducing property of the metal. ()
Tip:×. The reducibility of metals is related to the difficulty of losing electrons, and has nothing to do with the number. For example, K loses 1 electron and becomes K+, Mg loses 2 electrons and becomes Mg2+. Mg loses more electrons than K, but the reducibility K>Mg .
(3) When the element is at its highest price, the substance must have strong oxidizing properties. ()
Tip:×. The +1 price of potassium is the highest, but the oxidizing property of K+ is very weak.
2. Oxidation-reduction reactions in production and life
[Situation·Thinking] In recent years, the number of medical beauty institutions has increased day by day. Due to the uneven level of various institutions and the mixed mix of medical practitioners, medical beauty disputes are increasing day by day. Criminals use toxic H3AsO3 as hair removal agents to harm customers.
In concentrated hydrochloric acid, the ionic equation for the reaction between H3AsO3 and SnCl2 is:
3SnCl2+12Cl-+2H3AsO3+6H+====2As+3 +6M.
(1) What substance is M in the above reaction equation?
Tip: From atomic conservation we know that M is H2O.
(2) Is the above reaction a redox reaction? If so, please indicate the reaction
Oxidants, reducing agents, oxidation products and reduction products should be included.
Tip: During the reaction, the valence of As decreases and the valence of Sn increases, which is a redox reaction; from the changes in the valence of elements during the reaction, we know that H3AsO3 is the oxidant, As is the reduction product, SnCl2 is the reducing agent, and ___ is the oxidation product.
Oxidizing agent and reducing agent PPT, part 2 content: Key capabilities and attainment of literacy
Knowledge points: Comparison of the oxidizing and reducing properties of substances
[Key points to clarify doubts]
(1) Judging based on the direction of the redox reaction
Oxidizing properties: oxidizing agent > oxidation product
Reducibility: reducing agent>reduction product
(2) Comparison based on the activity order of elements
(3) Judge according to reaction conditions
When different oxidants (or reducing agents) react with the same reducing agent (or oxidizing agent), the easier the reaction is to proceed, the stronger the oxidizing (or reducing) properties of the corresponding oxidizing agent (or reducing agent), and vice versa. like:
①MnO2+4HCl (concentrated) MnCl2+Cl2↑+2H2O
2KMnO4+16HCl(concentrated)====2KCl+2MnCl2+5Cl2↑+8H2O
Oxidizing properties: KMnO4>MnO2.
②The reaction conditions of Na, Mg, Al elemental substances and H2O are as follows. Na reacts violently with cold water, Mg only reacts when heated, and Al is difficult to react under heating conditions, so the reduction property is: Na>Mg>Al.
【Think·Discussion】
(1) The stronger the oxidation property, the more electrons are gained; the stronger the reduction property, the more electrons are lost. Is this correct? Please give an example to explain the reason.
Hint: Wrong. Oxidizing Cl2>S, but when the two react with Fe, each Cl in Cl2 gets one electron to oxidize iron to FeCl3, and S gets two electrons to oxidize Fe to FeS; reducing Na>Fe, but when the reaction occurs, Na Lose an electron.
(2) Among compounds of the same element with different valence states, does the higher the valence of the element, the stronger the oxidizing property of the substance? Please give an example.
Hint: No. In general, the higher the valence of an element in a substance, the stronger its oxidizing property. However, this is not necessarily the case for some substances. For example, the valence of chlorine in HClO4 is +7, which is higher than the +1 valence in HClO, but the oxidizing property of HClO4 is weaker than that of HClO. , because the oxidability of a substance is not only related to the valence, but also to the stability of the substance itself.
Keywords: PPT courseware for high school chemistry compulsory course 1 from the People's Education Press is available for free download, oxidizing agent and reducing agent PPT download, redox reaction PPT download, .PPT format;
For more information about the PPT courseware "Oxidants and Reducing Agents in Redox Reactions", please click the Oxidizing and Reducing Agents ppt tag in Redox Reaction ppt.
"Redox Reactions" PPT courseware of elements and the world of matter (Application of redox reactions in Lesson 3):
"Redox Reactions" Elements and the World of Matter PPT Courseware (Application of Redox Reactions in Lesson 3) Part One: Learning Objectives Course Standards 1. Be able to predict the oxidizing or reducing properties of substances based on the rising and falling trends in valence of core elements. 2. Know that oxidation also...
"Oxidation-Reduction Reaction" Elements and the World of Matter PPT Courseware (Lesson 2 Oxidizing Agents and Reducing Agents):
"Redox Reactions" Elements and the World of Matter PPT Courseware (Lesson 2 Oxidizing Agents and Reducing Agents) Learning Objectives Course Standards 1. Know common oxidizing agents and reducing agents. Understand the basic laws of redox reactions. 2. Based on the principle of redox reaction, predict substances...
"Redox Reactions" PPT courseware of elements and the world of matter (understanding redox reactions in the first lesson):
"Redox Reactions" Elements and the World of Matter PPT Courseware (Understanding Redox Reactions in Lesson 1) Part One Content: Learning Objectives Course Standards 1. Understand that chemical reactions with changes in the valence of elements are redox reactions, and understand the essence of redox reactions. electricity..
File Info
Update Time: 2024-09-26
This template belongs to Chemistry courseware People's Education Press High School Chemistry Compulsory Course I industry PPT template
"Oxidant and Reducing Agent" Redox Reaction PPT Courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Oxidant and Reducing Agent" Redox Reaction PPT Courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Oxidant and Reducing Agent" Redox Reaction PPT Courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview