| Category | Format | Size |
|---|---|---|
| People's Education Press High School Chemistry Compulsory Course 2 | pptx | 6 MB |
Description
"Development and Utilization of Natural Resources" Chemistry and Sustainable Development PPT Courseware (Lesson 1)
Part One: Core Competency Development Goals
1. Understand the general principles of metal smelting, master the general methods of metal smelting with different reactivity, form a knowledge model of metal smelting methods, and cultivate the ability of "evidence reasoning and model cognition".
2. Understand the main components of seawater, understand seawater resources and the main methods of development and utilization, master the chemical reaction principles and experimental methods of extracting bromine from seawater, and cultivate "macro recognition and change concepts."
3. Cultivate the spirit of “scientific inquiry” and enhance the sense of social responsibility through understanding of the principles of metal smelting and the comprehensive utilization of seawater resources.
Development and Utilization of Natural Resources PPT, Part 2: 01 Development and Utilization of Metal Minerals
Knowledge sorting
1. The existence form of metallic elements in nature
2. General principles of metal smelting
(1)Basic principle: ______ out metallic elements from their compounds.
(2) Expression method: Mn++ne-===M.
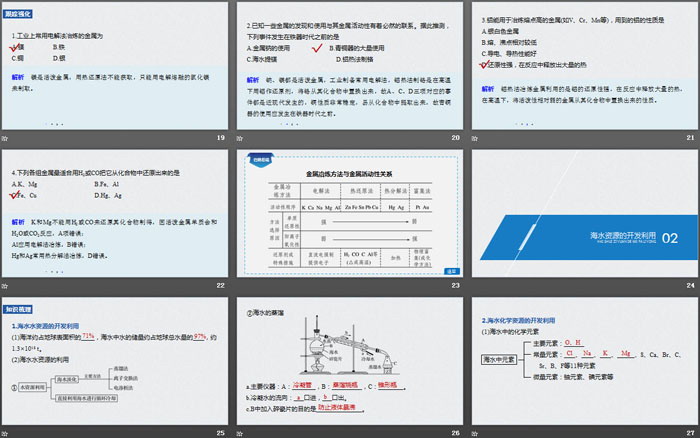
3. General methods of metal smelting
(1) Basis: The reactivity of metals is different, and the difficulty of reducing them from the combined state to the free state is different.
(2) Write the chemical equation for smelting metals using the following methods
①Thermal decomposition method: thermal decomposition of HgO: 2HgO == 2Hg+O2↑
Heating decomposition of Ag2O: 2Ag2O == 4Ag+O2↑
②Thermal reduction method: H2 reduction of CuO: CuO+H2 == Cu+H2O
Blast furnace ironmaking: Fe2O3+3CO == 2Fe+3CO2
Thermite reaction: 2Al+Fe2O3 == 2Fe+Al2O3
③Electrolysis method: smelting sodium:___________________________
Smelting Magnesium:_______________________________
Smelting Aluminum:_______________________________
④Other methods: Hydrometallurgical copper smelting: __________________
4. Rational development and utilization of metal mineral resources
(1) Improve the utilization rate of metal minerals.
(2) Develop environmentally friendly and efficient metal smelting methods.
(3) Prevent metal corrosion.
(4) Strengthen the recycling and reuse of scrap metal.
(5) Use other materials instead of metal materials.
True or false
(1) All metal elements must be obtained through chemical reactions ()
(2) When using compounds to smelt metals, the metal elements must undergo a reduction reaction ()
(3) The more reactive metal elements are, the later they are used by humans ()
(4) When smelting metals by thermal reduction method, H2 can be used to reduce Mg from the compound ()
(5) The essence of the thermite reaction is to use aluminum to replace metals that are less reactive than aluminum from their compounds ()
deep thinking
1. Thermite reaction can be used to weld large-section rails in the field. The principle is 2Al+Fe2O3 == Al2O3+2Fe. A group used the instruments and reagents as shown in the figure to explore the thermite reaction.
The experimental phenomena are recorded as follows: ① Violent reaction, sparks shooting out. ② A large amount of heat is released, the lower end of the paper funnel is burned through, and a red-hot molten substance falls onto the fine sand, and turns into a black solid after cooling. Based on the above description, answer the following questions:
(1) Thermite reaction requires high temperature conditions. Is continuous heating required?
Tip: Thermite reaction is an exothermic reaction, and the heat released can keep the reaction going, so continuous heating is not required.
(2) What are the functions of magnesium strips and potassium chlorate in the experiment? What is the action that triggers the reaction?
Tip: The magnesium bar is an igniter, and potassium chlorate is a combustion accelerant. The magnesium bar is ignited, causing the potassium chlorate to decompose and release oxygen, causing the magnesium bar to burn more violently and release more heat per unit time, thereby triggering the reaction between Al and Fe2O3.
(3) What is the purpose of spreading a small amount of fine sand in the evaporating dish?
Tips: One is to prevent the evaporating dish from exploding, and the other is to prevent the generated molten material from splashing out.
(4) According to the reaction principle, what is the best mass ratio of Fe2O3 and Al?
Tip: In order to ensure that both Fe2O3 and Al react completely, it is best to control the mass ratio of the two at 3:1. [m(Fe2O3):m(Al)=160:(2×27)≈3:1]
Tracking reinforcement
1. The metals commonly used in industry to be smelted by electrolysis are
A.Magnesium B.Iron
C.Copper D.Silver
2. It is known that the discovery and use of some metals are inevitably related to their metal activity. It is speculated that the following events occurred before the Iron Age:
A. The use of metallic sodium B. The extensive use of bronzes
C. Extraction of magnesium from seawater D. Preparation of chromium by thermite method
3. Aluminum can be used to smelt metals with high melting points (such as V, Cr, Mn, etc.). The properties of the aluminum used are
A.Silver white metal
B. Melting and boiling points are relatively low
C. Good electrical and thermal conductivity
D. It is highly reducing and releases a lot of heat during the reaction.
4. Which of the following groups of metals is best suited to be reduced from compounds using H2 or CO?
A.K、Mg B.Fe、Al
C.Fe, Cu D.Hg, Ag
Development and Utilization of Natural Resources PPT, Part Three: 02 Development and Utilization of Sea Water Resources
Knowledge sorting
1. Development and utilization of sea water resources
(1) The ocean occupies about _____ of the earth’s surface area, and the water reserves in seawater account for about _____ of the earth’s total water, about 1.3×1018 t.
(2) Utilization of sea water resources
②Distillation of sea water
a. Main instruments: A: __________, B: __________, C: __________.
b. The flow direction of condensate water: inlet from _____ port and out from _____ port.
c.The purpose of adding broken porcelain pieces to B is_______________.
2. Development and utilization of seawater chemical resources
(1) Chemical elements in seawater
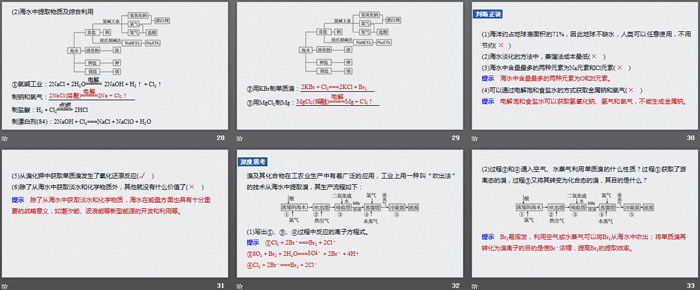
(2) Extraction of substances from seawater and comprehensive utilization
①Chlor-alkali industry: 2NaCl+2H2O == 2NaOH+H2↑+Cl2↑
Sodium and chlorine production: ____________________________
Making hydrochloric acid: H2+Cl2==2HCl
Preparing bleach (84): 2NaOH+Cl2===NaCl+NaClO+H2O
②Preparation of elemental bromine from KBr: ____________________
③Preparation of Mg from MgCl2: __________________
True or false
(1) The ocean accounts for about 71% of the earth’s surface area, so the earth is not short of water and humans can use it as they wish without saving ()
(2) Among seawater desalination methods, distillation is the lowest cost ()
(3) The two most abundant elements in seawater are Na and Cl ()
(4) Metal sodium and chlorine can be obtained by electrolyzing saturated salt water ()
(5) Obtaining elemental bromine from potassium bromide undergoes a redox reaction ()
(6) Except for obtaining fresh water and chemicals from sea water, there is no other value ()
deep thinking
Bromine and its compounds are widely used in industrial and agricultural production. In industry, a technology called "blowing method" is used to extract bromine from seawater. The production process is as follows:
(1) Write the ionic equations for the reactions in processes ①, ③, and ④.
(2) What properties of elemental bromine are utilized by introducing air and water vapor in processes ② and ④? Process ① obtains free bromine, and process ③ converts it into combined bromine. What is its purpose?
(3) Process ⑤ During the distillation process, the temperature is controlled at 80~90 ℃. Too high or too low temperature is not conducive to production. What is the reason?
Tracking reinforcement
1. In the comprehensive utilization of seawater resources, the substances that can be obtained from seawater without chemical changes are
A. Bromine and iodine elements B. Sodium and magnesium elements
C. Salt, fresh water D. Caustic soda, soda ash
2. Our country has always attached great importance to the comprehensive utilization of seawater resources. Which of the following statements about the comprehensive utilization of seawater is correct?
A. Using tidal power to generate electricity is to convert chemical energy into electrical energy
B. Chemical changes occur during the evaporation of seawater to produce sea salt.
C. Seawater contains bromine element, and elemental bromine can be obtained only through physical changes.
D. MgCl2 can be obtained from seawater, and Mg can be produced by electrolysis of molten MgCl2.
Keywords: PPT courseware for high school chemistry compulsory course 2 from the People's Education Press is available for free download, development and utilization of natural resources PPT download, chemistry and sustainable development PPT download, development and utilization of metal minerals and sea water resources PPT download, .PPT format;
For more information about the PPT courseware "Chemistry and Sustainable Development, Development and Utilization of Natural Resources, Metal Minerals, and Sea Water Resources," please click on the Chemistry and Sustainable Development PPT, Development and Utilization of Natural Resources, PPT Metal Minerals, and Sea Water Resources, Development and Utilization PPT tags.
"Development and Utilization of Natural Resources" Chemistry and Sustainable Development PPT Courseware (Micro Topic 10):
"Development and Utilization of Natural Resources" Chemistry and Sustainable Development PPT Courseware (Micro Topic 10) Part One Content: New Course Introduction 1. Structure of process flow questions: main line main product, branch by-products, and return to cycle. 2. Common methods for pretreatment of raw materials (1)..
"Development and Utilization of Natural Resources" Chemistry and Sustainable Development PPT Courseware (Lesson 2):
"Development and Utilization of Natural Resources" Chemistry and Sustainable Development PPT Courseware (Lesson 2) Part One Content: Core Competency Development Goals 1. Understand the composition of coal, oil, and natural gas and the significance of comprehensive utilization, and improve the ability of macroscopic identification and microscopic analysis ability. 2. Understand..
"Development and Utilization of Natural Resources" Chemistry and Sustainable Development PPT (Lesson 2):
"Development and Utilization of Natural Resources" Chemistry and Sustainable Development PPT (Lesson 2) Part One Content: Foundation for Essential Knowledge Literacy 1. Comprehensive Utilization of Coal 1. Composition of Coal 2. Comprehensive Utilization of Coal [Micro Thoughts] If from What should be used to separate aromatic hydrocarbons from coal tar?
File Info
Update Time: 2024-07-02
This template belongs to Chemistry courseware People's Education Press High School Chemistry Compulsory Course 2 industry PPT template
"Development and Utilization of Natural Resources" Chemistry and Sustainable Development PPT Courseware (Lesson 1) Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Development and Utilization of Natural Resources" Chemistry and Sustainable Development PPT Courseware (Lesson 1) is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Development and Utilization of Natural Resources" Chemistry and Sustainable Development PPT Courseware (Lesson 1), due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview