People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
People's Education Press High School Chemistry Compulsory Course 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| People's Education Press High School Chemistry Compulsory Course 2 | pptx | 6 MB |
Description
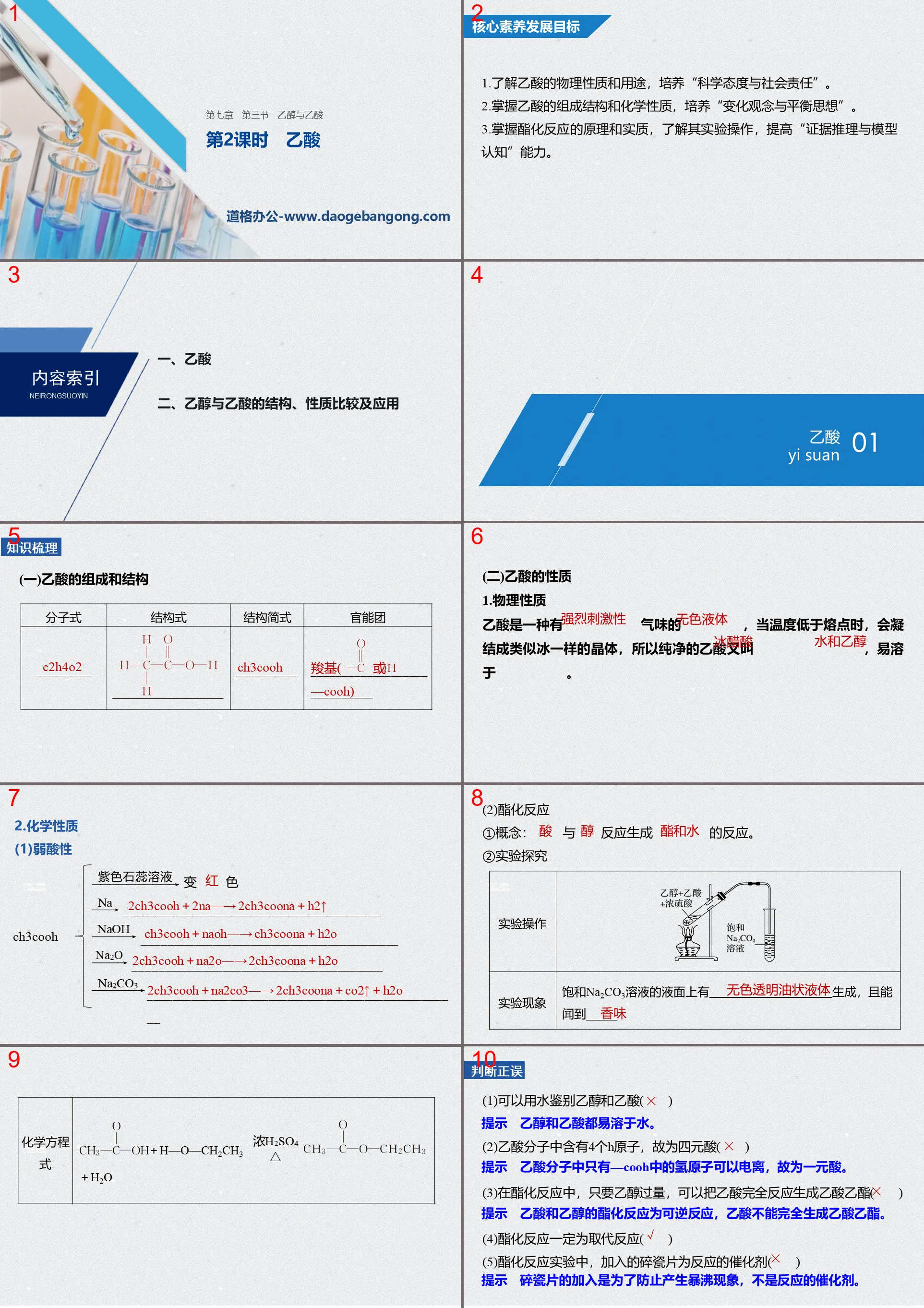
"Ethanol and Acetic Acid" Organic Compounds PPT Courseware (Lesson 2 Acetic Acid)
Part One: Core Competency Development Goals
1. Understand the physical properties and uses of acetic acid, and cultivate "scientific attitude and social responsibility".
2. Master the composition structure and chemical properties of acetic acid, and cultivate "the concept of change and the idea of balance."
3. Master the principle and essence of esterification reaction, understand its experimental operation, and improve the ability of "evidence reasoning and model cognition".
Ethanol and acetic acid PPT, Part 2 content: 01 Acetic acid
Knowledge sorting
(1) Composition and structure of acetic acid
(2) Properties of acetic acid
1.Physical properties
Acetic acid is a _____________ with a _____________ smell. When the temperature is lower than the melting point, it will condense into ice-like crystals. Therefore, pure acetic acid is also called _____________ and is easily soluble in _____________.
2. Chemical properties
(1) Weak acidity
(2)Esterification reaction
①Concept: The reaction between __________ and __________ produces __________.
②Experimental research
True or false
(1) Ethanol and acetic acid can be distinguished with water ()
(2) The acetic acid molecule contains 4 H atoms, so it is a tetrabasic acid ()
(3) In the esterification reaction, as long as there is an excess of ethanol, acetic acid can be completely reacted to form ethyl acetate ()
(4) The esterification reaction must be a substitution reaction ()
(5) In the esterification reaction experiment, the added broken porcelain pieces serve as the catalyst for the reaction ()
deep thinking
1. In daily life, vinegar can be used to eliminate scale in kettles (the main component is calcium carbonate). What are the properties of acetic acid? Write the corresponding chemical reaction equation.
2. Design experiments to prove: acidity: hydrochloric acid > acetic acid > carbonic acid, and write the corresponding chemical equation.
3. The laboratory uses the device as shown in the figure to prepare ethyl acetate.
Try answering the following questions:
(1) When adding drugs, why not add concentrated H2SO4 first, and then add ethanol and acetic acid?
(2) Why can’t the end of the catheter be inserted into the saturated Na2CO3 solution?
(3) What is the function of concentrated H2SO4? What is the function of saturated Na2CO3 solution?
(4) Ethanol containing the tracer atom 18O is used to participate in the reaction. Among the products generated, only ethyl acetate contains 18O. What bonds are broken in the acetic acid and ethanol molecules during the esterification reaction?
Tracking reinforcement
1. Which of the following statements about the properties of acetic acid is incorrect?
A. Acetic acid is more acidic than carbonic acid, so it can react with carbonate solution to produce CO2 gas.
B. Acetic acid is acidic, so it can react with sodium to release H2
C. The acetic acid molecule contains carbon-oxygen double bonds, so it can discolor bromine water.
D. Acetic acid condenses into ice crystals when the temperature is lower than 16.6 ℃
2. Esterification reaction is an important type of reaction in organic chemistry. Which of the following is an incorrect understanding of esterification reaction?
A. The product of esterification reaction is only ester
B. The esterification reaction is a reversible reaction and has limitations.
C. During the esterification reaction, in order to prevent bumping, several pieces of broken porcelain should be added in advance.
D. Esterification reaction is a substitution reaction
3. The main steps for preparing ethyl acetate in the laboratory are as follows:
①Add a mixed solution of 3 mL ethanol, 2 mL concentrated sulfuric acid and 2 mL acetic acid into test tube A (as shown in the picture).
② Connect the device as shown in the figure (the device is airtight), add the mixed liquid, and heat evenly over low heat for 3 to 5 minutes.
③After a certain amount of product has been collected in test tube B, stop heating, remove test tube B, shake vigorously, and then let it stand for stratification.
④ Separate the ethyl acetate layer, wash and dry.
(1) The role of concentrated sulfuric acid in the reaction is ____________________.
(2) The function of saturated sodium carbonate solution in the above experiment is ________(fill in the letter).
A. Neutralize acetic acid and ethanol
B. Neutralize the acetic acid and absorb part of the ethanol
C. The solubility of ethyl acetate in saturated sodium carbonate solution is smaller than that in water, which is conducive to stratification.
D. Accelerate the formation of ester and increase its yield
Ethanol and acetic acid PPT, Part 3 content: 02 Structure, property comparison and application of ethanol and acetic acid
Knowledge sorting
1. The bond breaking rules of ethanol in chemical changes
① Bond breaking: React with ______, react with acetic acid ______.
The ① bond and the ③ bond are broken at the same time: ____________ reaction occurs.
2. The bond-breaking rules of acetic acid in chemical changes
①The key is broken: ________ is displayed.
②Bond breaking: ________ reaction occurs.
3. In application, if the molecular structure of the organic matter in question contains -OH or _______________, it can be judged and deduced based on the properties of ethanol and acetic acid.
True or false
(1) Both ethanol and acetic acid molecules contain O—H covalent bonds, so their aqueous solutions are acidic ()
(2) When ethanol and acetic acid undergo an esterification reaction under certain conditions, the ethanol molecules remove hydroxyl groups and the acetic acid molecules remove hydrogen atoms ()
(3) Acetic acid molecules also contain C—H covalent bonds and O—H covalent bonds, so acetic acid can also undergo catalytic oxidation reactions with oxygen ()
Tracking reinforcement
1. (2019·Tianjin Senior High School Test) The simplified structural formula of a certain organic compound is ______________. Which of the following statements about this organism is incorrect?
A. Can react with NaHCO3 and release carbon dioxide
B. Can undergo addition reaction with HCl under the action of catalyst
C. Cannot discolor acidic KMnO4 solution and bromine water
D. Under the condition of concentrated sulfuric acid as a catalyst, it can react with ethanol to form ester.
2. (2018 Shanxi Jinzhong Senior High School Entrance Examination Adaptability Survey) Linalool and nerol are the key substances that determine the sweet aroma of tea flowers. The structures of linalool and nerol are shown in the figure. Which of the following statements is correct?
A. The molecular formula of nerolid alcohol is C15H28O
B. Linalool and nerolid alcohol are isomers of each other
C. The products of linalool and nerolid alcohol and H2 are homologues of each other
D. Both can undergo substitution reactions, addition reactions, and reduction reactions, but cannot undergo oxidation reactions.
3. The simplified structural formula of an organic compound is shown in the figure. Which of the following statements is incorrect?
A. The reaction of 1 mol of this organic substance and excess metallic sodium can produce up to 1.5 mol of H2
B. The ratio of the substances that consume the most Na, NaOH, and NaHCO3 is 3:2:2
C. You can use acidic KMnO4 solution to test the carbon-carbon double bonds in it
D. This substance can be oxidized to organic matter containing -CHO under the action of a catalyst
Keywords: Free download of PPT courseware for high school chemistry compulsory course 2 from the People's Education Press, download of organic compounds PPT, download of ethanol and acetic acid PPT, download of acetic acid PPT, .PPT format;
For more information about the "Organic Compounds Ethanol and Acetic Acid Acetic Acid" PPT courseware, please click the Organic Compounds PPT Ethanol and Acetic Acid PPT Acetic Acid PPT tab.
"Ethanol and Acetic Acid" Organic Compounds PPT courseware (micro-topic analysis and exploration of the properties of multi-functional organic compounds):
"Ethanol and Acetic Acid" Organic Compounds PPT Courseware (Micro Topic Analysis and Exploration of the Properties of Multifunctional Organic Compounds) Tracking Training 1. The simplified structural formula of aromatic compound M is ___________. The correct statement about organic compound M below is A. The molecular formula is C10..
"Ethanol and Acetic Acid" Organic Compounds PPT Courseware (Classification of Functional Groups and Organic Compounds in Lesson 3):
"Ethanol and Acetic Acid" Organic Compounds PPT Courseware (Classification of Functional Groups and Organic Compounds in Lesson 3) Part One Content: Core Competency Development Goals 1. Understand the basis for classifying organic matter according to functional group categories, and understand the relationship between the transformation between functional groups and substance categories ..
"Ethanol and Acetic Acid" Organic Compounds PPT Lesson (Lesson 1 Ethanol):
"Ethanol and Acetic Acid" Organic Compounds PPT Lesson (Lesson 1 Ethanol) Part One Content: Core Competency Development Goals 1. Master the molecular structure and chemical properties of ethanol, understand the concepts of hydrocarbon derivatives and functional groups, and cultivate the concept of change and balance thinking . 2. Understand..
File Info
Update Time: 2024-09-30
This template belongs to Chemistry courseware People's Education Press High School Chemistry Compulsory Course 2 industry PPT template
"Ethanol and Acetic Acid" Organic Compounds PPT Courseware (Lesson 2 Acetic Acid) Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Ethanol and Acetic Acid" Organic Compounds PPT Courseware (Lesson 2 Acetic Acid) is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Ethanol and Acetic Acid" Organic Compounds PPT Courseware (Lesson 2 Acetic Acid), due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview