People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Hunan Education Edition Ninth Grade Chemistry Volume 1
Cantonese Education Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 2
People's Education Press High School Chemistry Compulsory Course 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
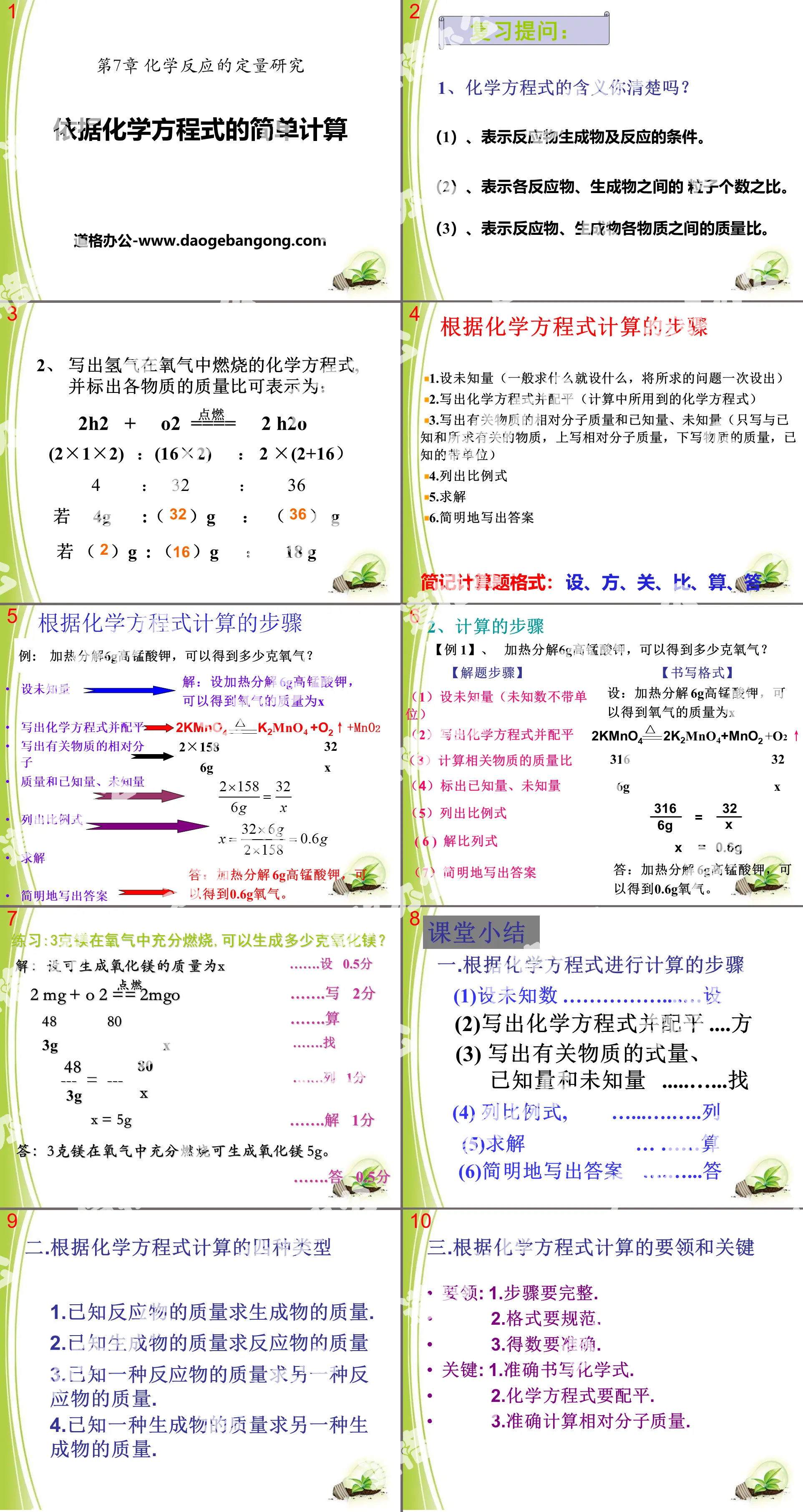
"Simple Calculation Based on Chemical Equations" Quantitative Research on Chemical Reactions PPT Courseware
Review questions:
1. Do you know the meaning of chemical equations?
(1), indicating the reactants, products and reaction conditions.
(2), represents the ratio of the number of particles between reactants and products.
(3), represents the mass ratio between reactants and products.
2. Write the chemical equation for the combustion of hydrogen in oxygen, and mark the mass ratio of each substance, which can be expressed as:
2H2 + O2 === 2H2O
(2×1×2): (16×2): 2×(2+16)
4:32:36
If 4g: ( )g: ( ) g
If ( )g : ( )g : 18 g
Calculation steps based on chemical equations
1. Set up the unknown quantity (generally set up whatever you are looking for, and set up the problem you are looking for at once)
2. Write chemical equations and balance them (chemical equations used in calculations)
3. Write the relative molecular mass, known quantities, and unknown quantities of the relevant substances (write only the substances related to the known and sought, write the relative molecular mass at the top, the mass of the substance at the bottom, and the known units)
4. List the proportional formulas
5. Solve
6. Write the answer concisely
Abbreviated calculation question format: Assume, Square, Guan, Compare, Calculate, Answer
Class summary
1. Steps for calculation based on chemical equations
(1) Assume the unknown number………………Suppose
(2)Write a chemical equation and balance it.... Square
(3) Write down the formula, known quantities, and unknown quantities of the relevant substances... Find
(4) Column proportional formula,………….…..column
(5) Solve... Calculate
(6)Write the answer concisely......Answer
2. Four types of calculations based on chemical equations
1. Find the mass of the product if the mass of the reactants is known.
2. If the mass of the product is known, find the mass of the reactant.
3. If the mass of one reactant is known, find the mass of the other reactant.
4. If the mass of one product is known, find the mass of the other product.
Through the above activities, the summary is as follows
1. To understand the calculation steps and methods, please pay attention to the following points:
(1) The calculation process must be standardized, and the steps can be summarized as: "One assumption, two writing, three searching, four columns, five solutions, and six answers."
(2) Each substitution amount refers to the quality of the pure substance.
If the substance is impure, it must be converted into the mass of the pure substance before it can be substituted into the chemical equation for calculation.
(3) The mass used in the calculation must be the mass of the substance actually participating in the reaction.
(4) During the calculation process, the units of each physical quantity must be consistent and unified, and the units must be brought into the calculation process.
2. Several errors that easily occur when calculating using chemical equations:
(1) The meaning of the question is unclear and the answer is not what is asked.
(2) Chemical equations are written incorrectly and are not balanced, which makes the calculation lose the correct basis.
(3) Carelessness, calculation errors when calculating the mass ratio of the required substances; or substitutions that appear to be pretentious.
(4) The units are not unified, and sometimes the volume is directly substituted for calculation.
(5) Substitute the amount of impure substances as the amount of pure substances.
Choose one
1. Which of the following phenomena can be explained by the law of conservation of mass ( )
A. mg water is heated to form mg water vapor
B. After drying, the mass of wet clothes becomes smaller.
C. The quality of coal ash left by coal combustion is less than that of raw coal.
D. Dissolve 10g of table salt in 70g of water to obtain 80g of table salt.
2. There must be no change before and after the chemical reaction ( )
① Number of atoms ② Number of molecules ③ Type of element ④ Type of substance ⑤ Type of atom
⑥The total mass of the substance
A. ①④⑥ B①③⑤⑥ C①④⑥ D②③⑤
3. The interaction between glucose and oxygen is the main reaction that constitutes biological respiration. The value of X is ( ) C6H12OX+6O2=6CO2+6H2O
A, 3 B, 6 C, 9 D, 12
4. Combustion of ethyl mercaptan: 2C2H5SH+9O2==4CO2+2X+6H2O, then X is ( )
A. H2SO4 B. SO3 C. SO2 D. CO2
5. Regarding the chemical equation CuO+H2==Cu+H2O, the information provided is incorrectly understood ( )
A. Indicates that CuO and H2 react to produce Cu and H2O under heating conditions.
B. Indicates that 1 CuO molecule reacts with 1 H2 molecule to generate 1 copper atom and 1 water molecule.
C. Indicates that the mass ratio of CuO, H2, Cu and H2O is 40:1:32:9
D. Indicates that the particle ratio of CuO, H2, Cu and H2O is 1:1:1:1
6. It is known that
A. 41g B. 32g C. 39g D. 10g
think about it
1. Under the guidance of the teacher, students in a chemistry interest group mixed and heated calamine (the main component is ZnCO3), red copper (the main component is Cu2O) and charcoal, and obtained a variety of substances, including zinc, copper, and gold. , carbon dioxide, which substance do you think cannot be obtained from it? Why?
2. The main components of the dark substance on the match head are KClO3, MnO2, and Sb2S3 (antimony sulfide). The side of the matchbox is coated with a layer of reddish-brown substance, whose main components are red phosphorus and glass powder. When a match is struck, an oxidation reaction occurs with the help of friction, oxygen and heat are released, and combustible materials are burned, producing white smoke and pungent smelling gases.
(1) How many reactions are there here?
(2) Write chemical equations for these reactions
Keywords: Teaching courseware for the quantitative study of chemical reactions, teaching courseware for simple calculations based on chemical equations, download the chemistry PPT courseware for the first volume of the ninth grade in the Beijing curriculum reform version, download the courseware for the ninth grade chemistry slideshow, download the PPT courseware for the quantitative study of chemical reactions, based on Simple calculation of chemical equations PPT courseware download, .PPT format;
For more information about the PPT courseware "Quantitative Study of Chemical Reactions Based on Simple Calculations of Chemical Equations", please click the "Quantitative Study of Chemical Reactions ppt Simple Calculations Based on Chemical Equations" ppt tag.
File Info
Update Time: 2024-10-02
This template belongs to Chemistry courseware Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Simple Calculation Based on Chemical Equations" Quantitative Research on Chemical Reactions PPT Courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Simple Calculation Based on Chemical Equations" Quantitative Research on Chemical Reactions PPT Courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Simple Calculation Based on Chemical Equations" Quantitative Research on Chemical Reactions PPT Courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview