People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
People's Education Press High School Chemistry Compulsory Course 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Hunan Education Edition Ninth Grade Chemistry Volume 2 | pptx | 6 MB |
Description
"Properties of Metals" Metals and Metal Materials PPT Courseware
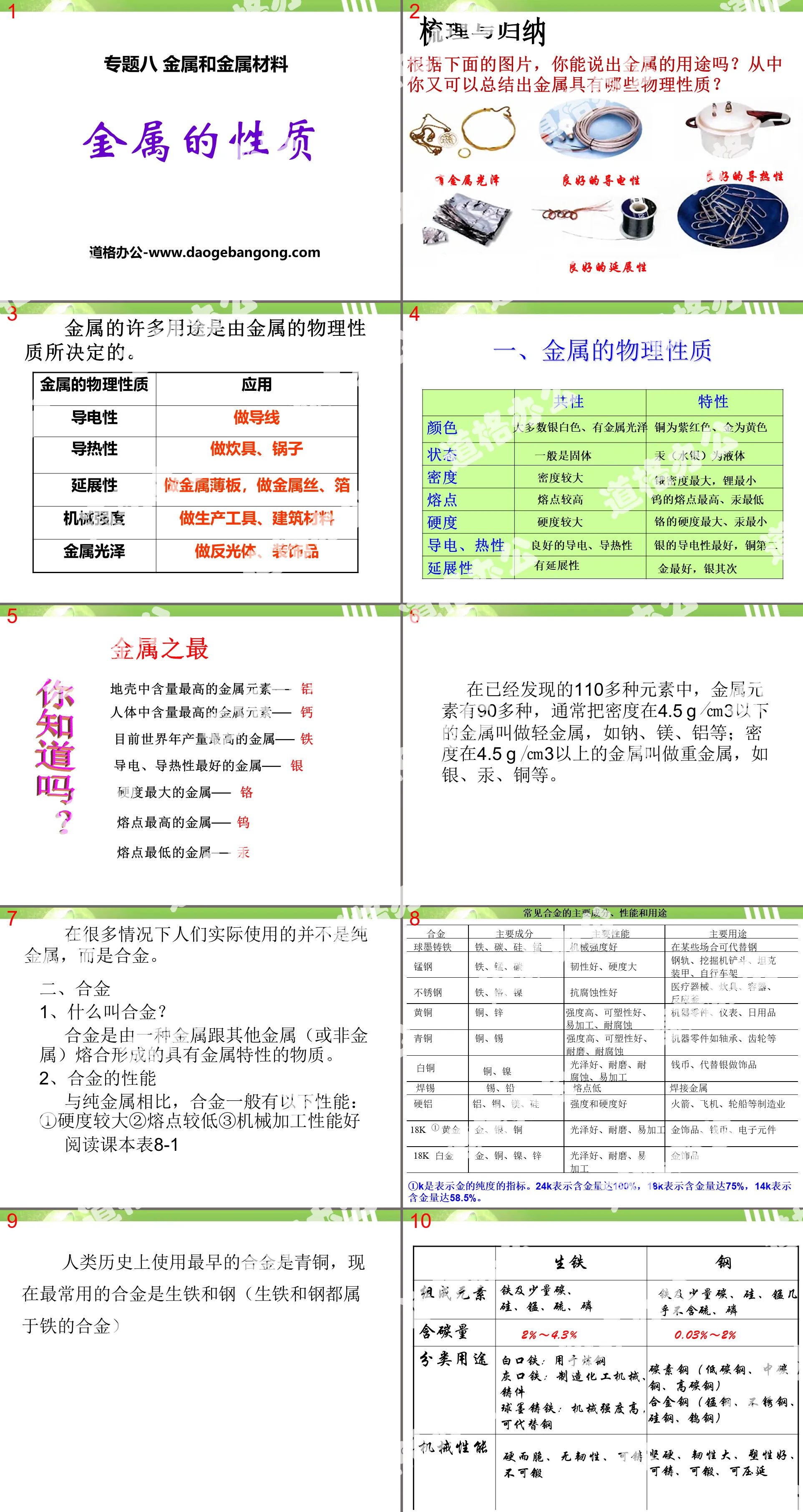
1. Physical properties of metals
The best of metal
The most abundant metal element in the earth’s crust─aluminum
The most abundant metal element in the human body─calcium
The metal with the highest annual output in the world - iron
The metal with the best electrical and thermal conductivity─Silver
The hardest metal─Chromium
The metal with the highest melting point─Tungsten
The metal with the lowest melting point─mercury
In many cases what people actually use is not pure metals, but alloys.
2. Alloy
1. What is alloy?
An alloy is a substance with metallic properties formed by the fusion of one metal with other metals (or non-metals).
2. Properties of alloy
Compared with pure metals, alloys generally have the following properties: ① Greater hardness ② Lower melting point ③ Good machining performance
Reading and Thinking
Read the content about alloys on pages P4~5 of the textbook, think about and answer the following questions.
1. The most commonly used metal material in the world is _____. Pig iron and steel are both alloys of __. They can be distinguished according to their carbon content. The one with more carbon is _____, and the one with less carbon is _____. Both pig iron and steel belong to ___________ (fill in the "pure substance or mixture").
2. If certain ______ or __________ are heated and fused in metal, substances with metallic characteristics, that is, alloys, can be produced.
3. Pure iron is ____, while pig iron is ___ than pure iron; stainless steel is not only ___ than pure iron, but its ________ performance is also much better than pure iron. Therefore, in daily life, industrial and agricultural production, and scientific research, what is often used extensively is not ________, but their _______.
4. Although there are only ___ kinds of pure metals that have been produced so far, there are ______ kinds of alloys made from these pure metals according to certain compositions and mass ratios.
3. Chemical properties of metals
(1) The shovel used for cooking is made of iron and usually has a wooden handle.
(2) Iron blocks can be made into iron wires or iron sheets
(3) When the tank truck is driving, the oil in the tank oscillates and generates static electricity, which is prone to fire hazards. Therefore, there is an iron chain mopping the ground at the rear of the tank truck.
think
1. Some metals can undergo displacement reactions with acids, but are the severity of the reactions the same?
2. Some metals can undergo displacement reactions with certain salt solutions. Why don't some reactions happen?
4. Explore the mobility of metals
Add a small amount of hydrochloric acid (or dilute sulfuric acid) of equal volume and concentration to each of the three test tubes. Add a clean magnesium bar, copper sheet and iron nail polished with sandpaper to each of the test tubes. Observe the reaction phenomenon and compare. The severity of its reaction.
Analysis and induction
1. In the above experiment, the metals that can react with acids are ____ and the metals that cannot react with acids are ____.
2. Among metals that can react with acids, the order of severity of reaction from strong to weak is ________.
3. Whether a metal can react with hydrochloric acid or dilute sulfuric acid to release hydrogen gas and the difference in the severity of the reaction can indicate the mobility of the metal. The more reactive the metal, the more violent the reaction with hydrochloric acid or dilute sulfuric acid. According to this, the order of metal activity from strong to weak among magnesium, copper, and iron is___________
practise:
1. Determine whether each of the following groups of substances can react? Write equations that can be reacted
(1) Silver and dilute hydrochloric acid
(2) Aluminum and dilute sulfuric acid
(3) Copper and zinc sulfate solution
(4) Zinc and copper sulfate solution
(5) Magnesium and silver nitrate solution
Test points:
1. Metal activity sequence and its applications;
2. Separation method of mixture;
3. Write chemical equations, text expressions, and ionization equations.
Analysis: Answer based on the principle of impurity removal. The principle of impurity removal is to remove impurities but not introduce new impurities.
Keywords: Metals and metallic materials teaching courseware, Properties of metals teaching courseware, Hunan Education Edition 9th grade chemistry PPT courseware download, Volume 2 chemistry, 9th grade chemistry slide courseware download, Metals and metallic materials PPT courseware download, Properties of metals PPT courseware download ,.PPT format;
For more information about the "Metals and Metal Materials Properties of Metals" PPT courseware, please click the Metals and Metal Materials ppt Properties of Metals ppt tag.
File Info
Update Time: 2024-10-01
This template belongs to Chemistry courseware Hunan Education Edition Ninth Grade Chemistry Volume 2 industry PPT template
"Properties of Metals" Metals and Metal Materials PPT Courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Properties of Metals" Metals and Metal Materials PPT Courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Properties of Metals" Metals and Metal Materials PPT Courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview