Fifth Grade Science Volume 1, Textbook Edition
Science Edition for Sixth Grade Science Volume 2
Science Edition for Sixth Grade Science Volume 1
Third Grade Science Volume 2, Textbook Edition
Third Grade Science Volume 1, Textbook Edition
Fourth Grade Science Volume 1, Textbook Edition
Fourth Grade Science Volume 2, Textbook Edition
Fourth-grade science volume 2 of the E-education edition
Qingdao Edition Fourth Grade Science Volume 2
Hunan Education Edition Fourth Grade Science Volume 1
E-education edition fifth grade science volume 1
E-education edition fifth grade science volume 2
E-education edition sixth grade science volume 1
Fifth Grade Science Volume 2, Textbook Edition
Zhejiang Education Edition Seventh Grade Science Volume 2
People's Education Press Fourth Grade Science Volume 2

| Category | Format | Size |
|---|---|---|
| Zhejiang Education Edition Eighth Grade Science Volume 2 | pptx | 6 MB |
Description
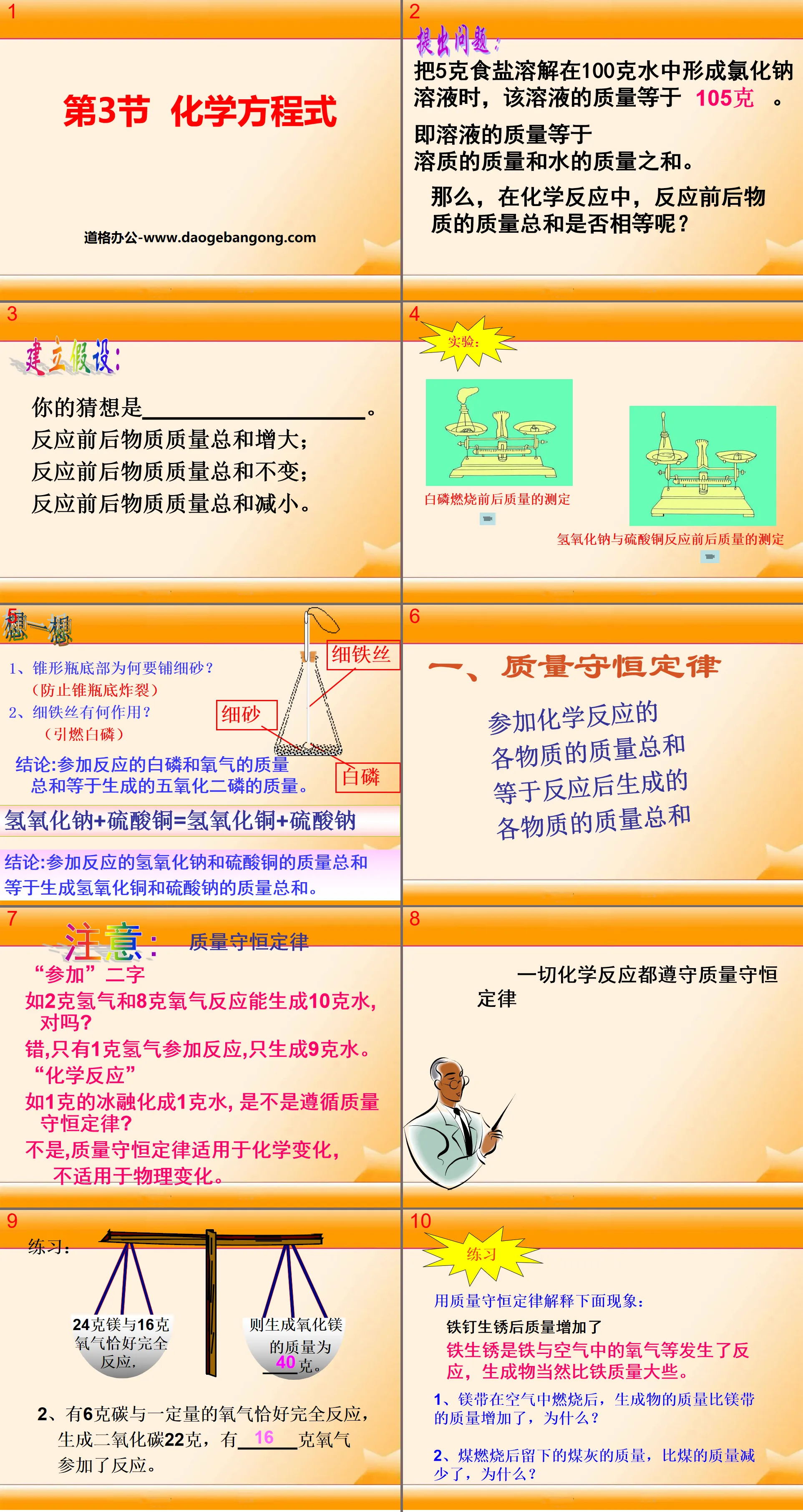
"Chemical Equations" PPT courseware
Part 1: Think about it
1. Why is the bottom of the Erlenmeyer flask covered with fine sand?
(Prevent the bottom of the Erlenmeyer flask from exploding)
2. What is the function of thin wire?
(ignites white phosphorus)
Conclusion: The sum of the masses of white phosphorus and oxygen participating in the reaction is equal to the mass of phosphorus pentoxide produced.
Sodium hydroxide + copper sulfate = copper hydroxide + sodium sulfate
Conclusion: The sum of the masses of sodium hydroxide and copper sulfate participating in the reaction is equal to the sum of the masses of copper hydroxide and sodium sulfate produced.
Chemical equation PPT, part 2 content: Law of conservation of mass
The sum of the masses of all substances participating in a chemical reaction is equal to the sum of the masses of all substances produced after the reaction.
Notice:
The word "participate"
For example, the reaction of 2 grams of hydrogen and 8 grams of oxygen can produce 10 grams of water, right?
Wrong, only 1 gram of hydrogen participates in the reaction, and only 9 grams of water are produced.
"chemical reaction"
For example, if 1 gram of ice melts into 1 gram of water, does it follow the law of conservation of mass?
No, the law of conservation of mass applies to chemical changes, not physical changes.
practise
Use the law of conservation of mass to explain the following phenomenon:
Iron nails gain mass after they rust
Iron rusts when iron reacts with oxygen in the air, and the product is of course heavier than iron.
1. After the magnesium ribbon is burned in the air, the mass of the product increases compared to the mass of the magnesium ribbon. Why?
2. The mass of coal ash left after coal is burned is less than the mass of coal. Why?
True or False:
1. After potassium permanganate is decomposed by heat, the remaining solid is lighter than the original reactant, which does not comply with the law of conservation of mass. ( )
2. After the candle burns, the remaining solid mass decreases, which does not comply with the law of conservation of mass. ( )
3. When a certain compound is completely decomposed by electricity, it will generate hydrogen (H2) and oxygen (O2). Try to infer that the component elements of this compound must contain ____ elements.
think:
How to use a formula to express a chemical reaction?
For example: carbon burns in oxygen to form carbon dioxide
This formula can indeed express the reactants, products and reaction conditions; however, it is inconvenient to write and is not universally applicable internationally. We have already learned chemical formulas, and we can use chemical formulas to express the composition of substances.
Chemical Equation PPT, Part 3: Chemical Equation
1. The concept of chemical equations
An equation that uses a chemical formula to express a chemical reaction is called a chemical equation.
2. Principles for writing chemical equations:
A. Based on objective facts
B. Follow the law of conservation of mass
3. Steps for writing chemical equations (taking phosphorus burning in oxygen as an example)
1) Based on the reaction facts, write the chemical formulas of the reactants and products on the left and right of the formula, connected with "——".
2) Balance the chemical equation (make the number of atoms before and after the reaction equal)
3) Change the horizontal line to an equal sign
4) Indicate: reaction conditions, product state (gas ↑ or precipitation ↓)
Chemical Equation PPT, Part 4: The Meaning of Chemical Equations
1) Macroscopically, it indicates what substances participate in the reaction and what substances are produced as a result.
Carbon and oxygen react under ignition conditions to produce carbon dioxide.
2) Microscopically, it represents the number ratio of atoms and molecules among various substances.
Particle number ratio: 1:1:1
3) Indicate under what conditions the reaction takes place.
4) Indicates the mass ratio between reactants and products.
Relative molecular mass ratio: 12:32:44
Every 12 parts by mass of carbon reacts with 32 parts by mass of oxygen to produce 44 parts by mass of carbon dioxide.
Chemical Equations PPT, Part 5: Practice:
Balance the following chemical equations
H2 + O2 —— H2O
Na + Cl2 - NaCl
KMnO4 -—— K2MnO4 + MnO2 + O2
Fe2O3 + HCl —— FeCl3 + H2O
Fe2O3 + H2SO4—— Fe2(SO4)3 + H2O
Ca(OH)2 + K2CO3 —— CaCO3 + KOH
Fe2O3 + CO —— Fe + CO2
Keywords: Zhejiang Education Edition eighth grade science PPT courseware for the second volume free download, chemical equation PPT download, .PPT format;
For more information about the "Chemical Equations" PPT courseware, please click the Chemical Equations ppt tab.
"Metal Materials" Iron Metal Materials PPT (Application of the quantity of matter in the calculation of chemical equations in Lesson 2):
"Metal Materials" Iron Metal Materials PPT (Application of the amount of matter in the calculation of chemical equations in Lesson 2) Part One Content: Learning Objectives Course Standards 1. Combined with chemical equations to understand the amount of matter, molar mass, gas molar volume, and matter Concepts such as quantity and concentration...
"Application of the Amount of Substance in the Calculation of Chemical Equations" Metal Materials PPT Download:
"The Application of the Amount of Substance in the Calculation of Chemical Equations" Metal Materials PPT Download Part One Content: Literacy Objective 1. Review and review the relationship between the quantity of substance n and the number of particles N, the mass of the substance m, the gas volume V, and the solution concentration c The calculation formula consolidates the amount of substance as...
"The Application of the Amount of Substance in the Calculation of Chemical Equations" Metal Materials PPT Courseware:
"The Application of the Amount of Substance in the Calculation of Chemical Equations" Metal Materials PPT Courseware Part One Contents: Foundation of Essential Knowledge Literacy 1. Relationship between the Amount of Substance and Each Physical Quantity 1. Graphical Relationship 2. Calculation Formula (1) has been Know the mass of the substance: n(B)=_______; ..
File Info
Update Time: 2024-11-15
This template belongs to science courseware Zhejiang Education Edition Eighth Grade Science Volume 2 industry PPT template
"Chemical Equations" PPT courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Chemical Equations" PPT courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Chemical Equations" PPT courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview