People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Hunan Education Edition Ninth Grade Chemistry Volume 2 | pptx | 6 MB |
Description
"Metal Corrosion and Protection" Metal and Metal Materials PPT Courseware
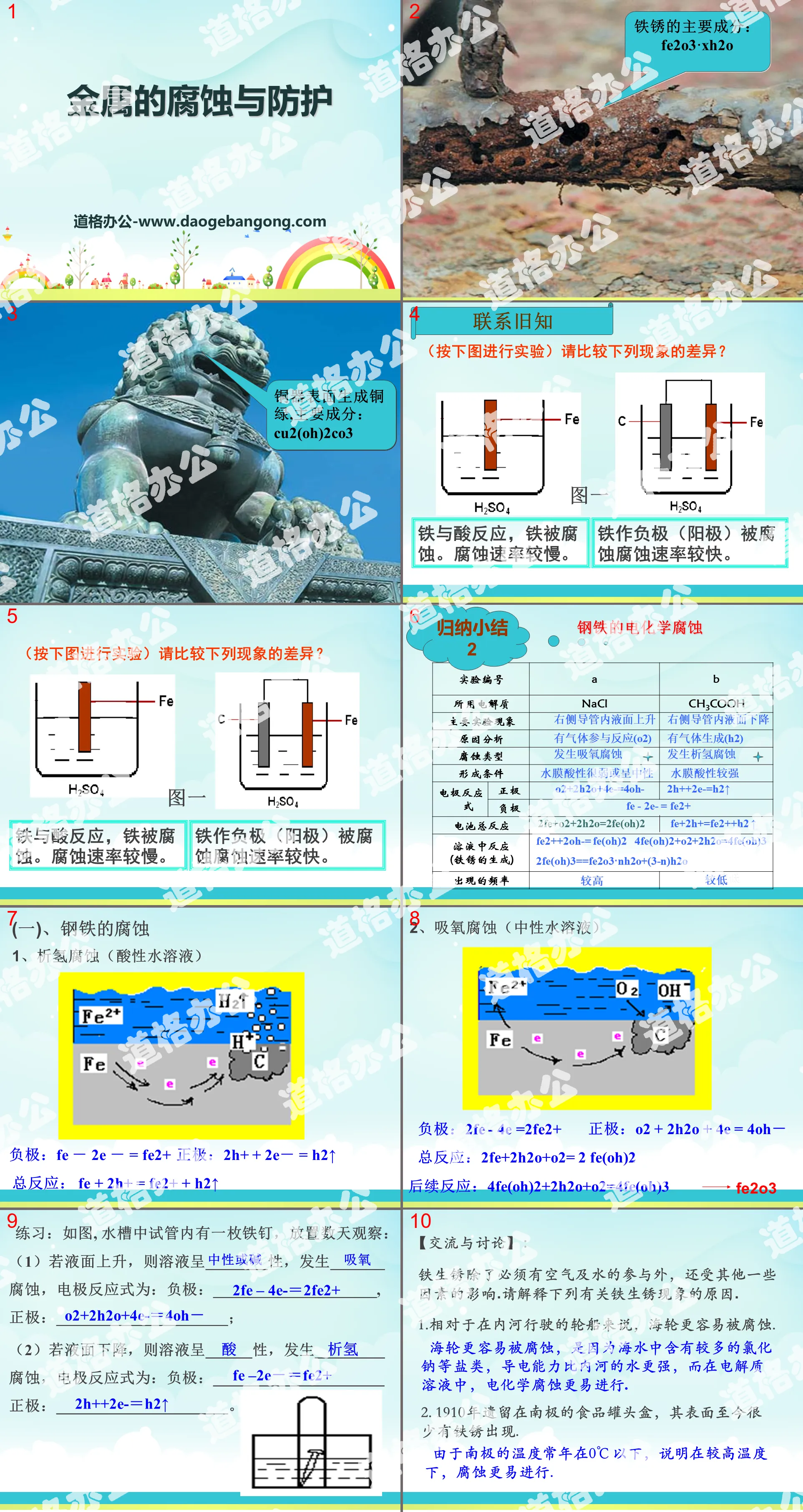
Contact old friends
Iron reacts with acid and the iron corrodes. Corrosion rate is slow.
Iron as the negative electrode (anode) is corroded at a faster rate.
Comparison of chemical corrosion and electrochemical corrosion of metals
The essence of metal corrosion
The process in which metal atoms lose electrons and are oxidized and consumed: M-ne-=Mn+
metal corrosion
Chemical corrosion
Corrosion caused by oxidation-reduction reactions in direct contact between metals and other substances
redox reaction occurs directly
No current is generated during the corrosion process
Metal is corroded directly
Electrochemical corrosion
Corrosion caused by the galvanic reaction of impure metals or alloys, causing the more active metal to lose electrons and be oxidized.
galvanic cell reaction occurs
There is current generated
More reactive metals are corroded
corrosion rate
Electrochemical corrosion>Chemical corrosion
connect
Often occur at the same time, but electrochemical corrosion is more common and more harmful
(1) Corrosion of steel
1. Hydrogen evolution corrosion (acidic aqueous solution)
Negative electrode: Fe - 2e -= Fe2+
Positive electrode: 2H+ +2e-= H2↑
Total reaction: Fe + 2H+ = Fe2+ + H2↑
2. Oxygen absorption corrosion (neutral aqueous solution)
Negative electrode: 2Fe - 4e =2Fe2+
Positive electrode: O2 + 2H2O + 4e = 4OH-
Total reaction: 2Fe+2H2O+O2= 2 Fe(OH)2
Subsequent reaction: 4Fe(OH)2+2H2O+O2=4Fe(OH)3
[Exchange and discussion]:
In addition to the participation of air and water, iron rusting is also affected by other factors. Please explain the following reasons for iron rusting.
1. Compared with ships traveling on inland rivers, seagoing ships are more susceptible to corrosion.
Sea-going vessels are more susceptible to corrosion because seawater contains more salts such as sodium chloride and has stronger electrical conductivity than inland water. In electrolyte solutions, electrochemical corrosion is more likely to occur.
2. There is very little rust on the surface of the food cans left in Antarctica in 1910.
Since the temperature in Antarctica is below 0°C all year round, corrosion is more likely to occur at higher temperatures.
3. The iron screws used to connect copper plates are easy to rust.
Since iron, copper and water vapor in the air form a primary battery, and iron serves as the negative electrode of the battery, it is more likely to corrode and rust.
4. When the exhaust gas sent out by the chemical plant is acidic gas, the iron products near the factory are prone to rust.
Because iron products are more susceptible to corrosion under acidic conditions than under neutral conditions.
2. Electrochemical protection of metals
Read and think:
1. What substances are related to the corrosion of iron? what is the essence
2. How to prevent iron from rusting? What's the easiest way?
Common methods in daily life to prevent iron from rusting by covering it with a protective layer:
1. Spray anti-corrosion paint on the surface of iron (such as bridges, cars, ships)
2. Oil and grease can be used to protect rotating parts made of metal in machines, such as gears.
3. Spray or wrap the metal surface with plastic (such as polyethylene, polyvinyl chloride, etc.).
4. Cover the surface of iron with a layer of enamel, such as face plates and other metal vessels.
5. Cover the surface of the iron with a layer of other metal (such as tin plating, galvanizing)
summary:
1. Two factors affecting metal corrosion.
Metal nature—whether the metal is active and pure
Medium – What substances do metals coexist with?
2. The nature of metal corrosion:
The process by which metal atoms lose electrons and become cations.
The simplest way to prevent metal from losing electrons is to isolate it from air and water and cover it with a protective layer.
3. Common protection methods for metal.
(1). Cover the metal surface with a protective layer.
a. Apply mineral grease, paint or cover with enameled plastic.
b. Plating anti-corrosion metal - electroplating, hot plating, spray plating.
c. Use chemical methods to form a dense oxide film on the surface, such as baking blue.
(2). Change the internal composition and structure of metal to enhance corrosion resistance, such as making it into stainless steel.
(3).Electrochemical protection method.
a. Cathodic protection method of sacrificial anode.
b. Cathodic protection method with impressed current.
Keywords: metal and metal materials teaching courseware, metal corrosion and protection teaching courseware, Hunan Education Edition ninth grade chemistry PPT courseware download, ninth grade chemistry slide courseware download, metal and metal materials PPT courseware download, metal corrosion and Download protective PPT courseware in .PPT format;
For more information about the "Metals and Metal Materials Metal Corrosion and Protection" PPT courseware, please click on the Metal and Metal Materials ppt Metal Corrosion and Protection ppt tag.
File Info
Update Time: 2024-11-22
This template belongs to Chemistry courseware Hunan Education Edition Ninth Grade Chemistry Volume 2 industry PPT template
"Metal Corrosion and Protection" Metal and Metal Materials PPT Courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Metal Corrosion and Protection" Metal and Metal Materials PPT Courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Metal Corrosion and Protection" Metal and Metal Materials PPT Courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview