People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Hunan Education Edition Ninth Grade Chemistry Volume 1
Cantonese Education Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| People's Education Press Ninth Grade Chemistry Volume 2 | pptx | 6 MB |
Description
"Common Acids and Bases" Acids and Bases PPT Courseware 2
teaching objectives
knowledge and abilities
Understand common acid-base indicators and their discoloration, learn about several common acids, and understand the corrosiveness of concentrated sulfuric acid.
Process and Method
Through independent inquiry experiments and anthropomorphic activity demonstrations, you can develop experimental skills, analytical abilities and the ability to communicate and cooperate with others.
Emotional attitudes and values
Look at the pros and cons of materials dialectically, grasp their properties, and use them rationally.
focus
1. Through experiments and common sense in daily life, understand the properties and uses of common acids and bases;
2. Through experiments, learn the properties of acids and bases, their reaction rules, and material preparation.
difficulty
Through comparative experiments, learn to identify acids, bases and other substances.
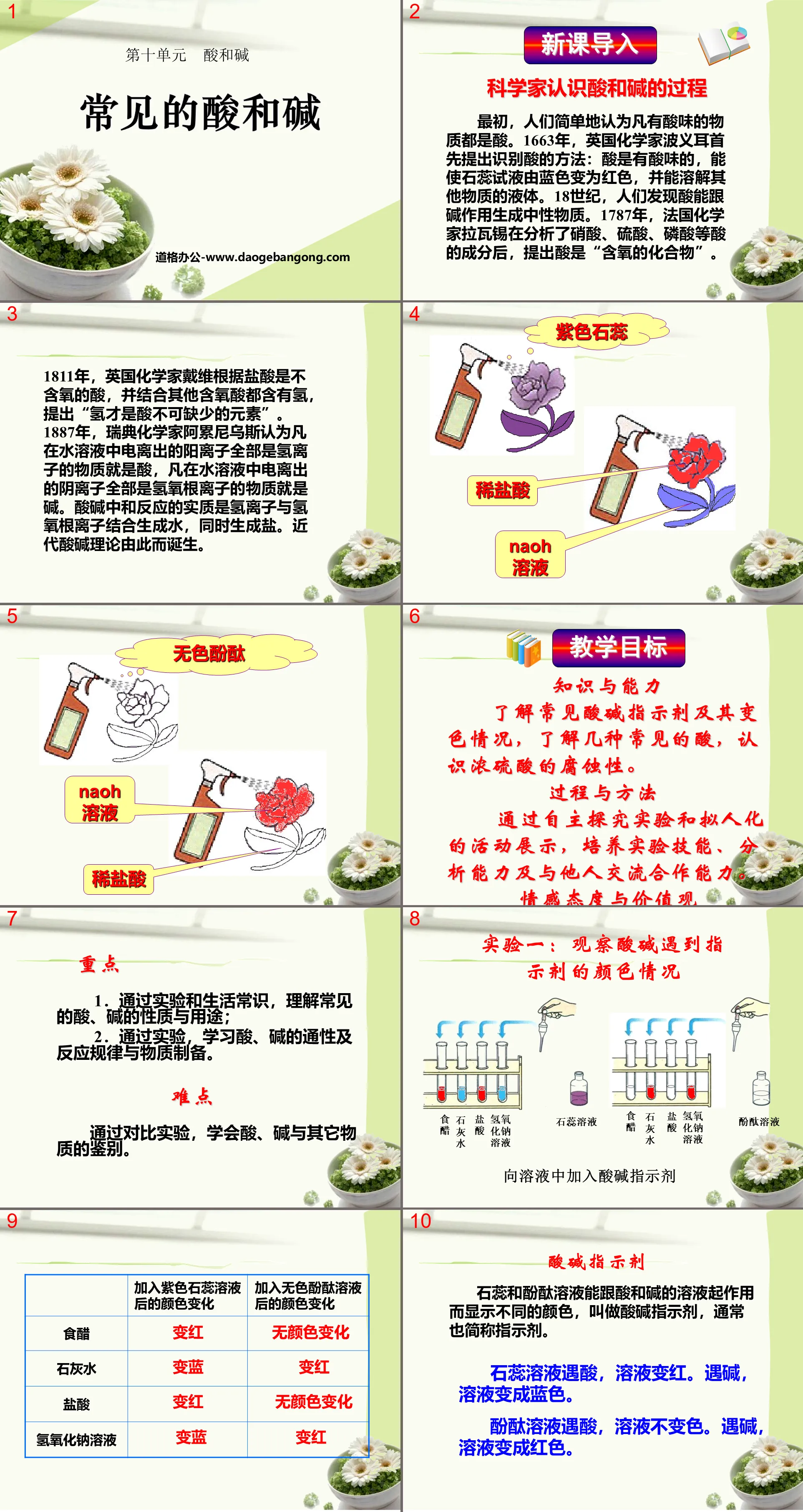
Acid-base indicator
Litmus and phenolphthalein solutions can react with acid and alkali solutions to show different colors. They are called acid-base indicators, often referred to as indicators.
When litmus solution encounters acid, the solution turns red. When encountering alkali, the solution turns blue.
When a phenolphthalein solution encounters acid, the solution does not change color. In the presence of alkali, the solution turns red.
1. Common acids
1. Several common acids
Overview of uses and dangers of hydrochloric acid (HCl)
(1) Hydrometallurgy for rare metals
(2) Used in organic synthesis
(3) Used in bleaching and dyeing industry
For example, hydrochloric acid is used for pickling after bleaching cotton cloth and neutralizing residual alkali after mercerizing cotton cloth. During the printing and dyeing process, some dyes are insoluble in water and need to be treated with hydrochloric acid to form soluble hydrochlorides before they can be used.
(4) Used for metal processing
For example, in the pre-plating treatment of steel parts, they are first washed with caustic soda solution to remove oil stains, and then soaked in hydrochloric acid; before metal welding, a little hydrochloric acid needs to be applied to the welding joint, etc., all of which use hydrochloric acid to dissolve metal oxides. A property to remove rust. In this way, the metal surface can be plated and welded firmly.
(5) Used in food industry
Hydrochloric acid has a catalytic effect and can promote protein hydrolysis.
(6) Used in the production of inorganic drugs and organic drugs
Hydrochloric acid is a strong acid that can react with certain metals, metal oxides, metal hydroxides, and most metal salts (such as carbonates, sulfites, etc.) to form hydrochlorides. Therefore, hydrochloric acid is used in the production of many inorganic drugs. Many organic drugs in medicine, such as novocaine and thiamine hydrochloride (preparations of vitamin B1), are also made from hydrochloric acid.
2. Common bases
1. Several common bases
①Sodium hydroxide is a white solid.
②Sodium hydroxide easily absorbs moisture when exposed to the air, and the surface becomes moist and gradually dissolves. This phenomenon is called deliquescence.
③Sodium hydroxide can be used as a desiccant for certain gases (such as H2, O2, etc.).
④Sodium hydroxide is easily soluble in water and releases a lot of heat when dissolved.
⑤ Commonly known as fire soda, caustic soda, and caustic soda.
⑥Sodium hydroxide is highly corrosive. If you accidentally get the alkali solution on your skin, rinse it with more water and then apply boric acid solution.
2. Properties of other bases
Calcium hydroxide[Ca(OH)2] CaO + H2O = Ca(OH)2
① Calcium hydroxide is corrosive, so please pay attention to safety when using it.
②The lime water used when testing carbon dioxide is an aqueous solution of calcium hydroxide.
③ Common alkalis include potassium hydroxide (KOH), ammonia (NH3·H2O), etc.
3. Chemical properties of bases
① Alkaline solution can turn purple litmus solution blue and colorless phenolphthalein solution red.
② Alkali can react with certain non-metal oxides:
2NaOH + CO2 = Na2CO3 + H2O
Note: Sodium hydroxide will deteriorate when placed in the air, so sodium hydroxide must be kept sealed.
Ca(OH)2+CO2=CaCO3↓+H2O (check CO2)
Class summary
1. Common acids
1. Several common acids: hydrochloric acid, sulfuric acid
2. The corrosiveness of concentrated sulfuric acid
3. chemical properties of acids
(1) The role of acid and indicator
Adding purple litmus solution turns red, adding colorless phenolphthalein solution does not change color.
(2) Reaction of acid and metal
(3) Reaction of acids and metal oxides
2. Common bases
1. Several common bases
Sodium hydroxide, calcium hydroxide
2. The corrosive nature of sodium hydroxide
3. Properties of other bases
4. Chemical properties of bases
(1) Alkaline solution can turn purple litmus solution blue and colorless phenolphthalein solution red.
(2) Alkali can react with certain non-metal oxides.
Class exercises
1. If there is a bottle of acidic colorless solution, pour a small amount into the test tube, and then add a few drops of phenolphthalein test solution. The solution will appear ____ color. If you want to make the solution in the test tube alkaline, you can use the ____________ method.
2. There is a bottle of alkaline colorless solution. Inject a small amount into the test tube, and then add a few drops of phenolphthalein test solution. The solution will appear ______ color. If you want to make the solution in the test tube acidic, you can use the ___________ method.
3. If the humid air passes through caustic soda solution, concentrated sulfuric acid and red-hot copper mesh in sequence, ______, ______ and _____ in the air will be removed in sequence, and the final remaining gas is mainly _____.
4. Among the following gases, which can be dried with both solid NaOH and concentrated H2SO4 ( )
A. CO2 B. HCl C. SO2 D. O2
5. Iron powder mixed with a small amount of copper oxide is added to a beaker containing dilute sulfuric acid. It reacts fully and some iron remains. After filtration, the solute contained in the filtrate is ( )
A. H2SO4 B. FeSO4 C. FeSO4 and CuSO4 D. H2SO4 and FeSO4
Keywords: Acids and bases teaching courseware, common acids and bases teaching courseware, New People's Education Edition ninth grade chemistry PPT courseware volume 2, ninth grade chemistry slide courseware download, acids and bases PPT courseware download, common acids and bases PPT Courseware download, .ppt format
For more information about the PPT courseware "Common Acids and Bases Acids and Bases", please click on the Common Acids and Bases ppt Acids and Bases ppt tab.
"Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware 3:
"Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware 3 1. Several common acids: 1. Physical properties of concentrated hydrochloric acid and concentrated sulfuric acid [Review Questions] 1. What are acids and bases? 2. What are the composition characteristics of acids and bases? Observe P11 experiments 1, 2, and 3 and complete the following...
"Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware 2:
"Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware 2 Preparation before class 1. Write the chemical formulas of common acids in life 2. Complete the following equations Mg Al Zn Fe; iron oxide, copper oxide and dilute respectively Chemical equation for the reaction of hydrochloric acid and dilute sulfuric acid. one..
"Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware:
"Several Common Acids and Bases" Initial Acids, Bases and Salts PPT Courseware Review before class 1. What kind of compound is called a base? Compounds in which all cations produced during ionization are H+ are called bases. 2. Which of the following compounds are bases? NaOH KOH Ca(OH)2 Ba(OH..
File Info
Update Time: 2024-10-03
This template belongs to Chemistry courseware People's Education Press Ninth Grade Chemistry Volume 2 industry PPT template
"Common Acids and Bases" Acids and Bases PPT Courseware 2 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Common Acids and Bases" Acids and Bases PPT Courseware 2 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Common Acids and Bases" Acids and Bases PPT Courseware 2, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview