"Classification and Transformation of Matter" PPT Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
| 文件名 如何下载使用 | 下载次数 | Download Points | 下载地址 |
|---|---|---|---|
| "Classification and Tran... | 14375次 | 0.00 | Free Download |
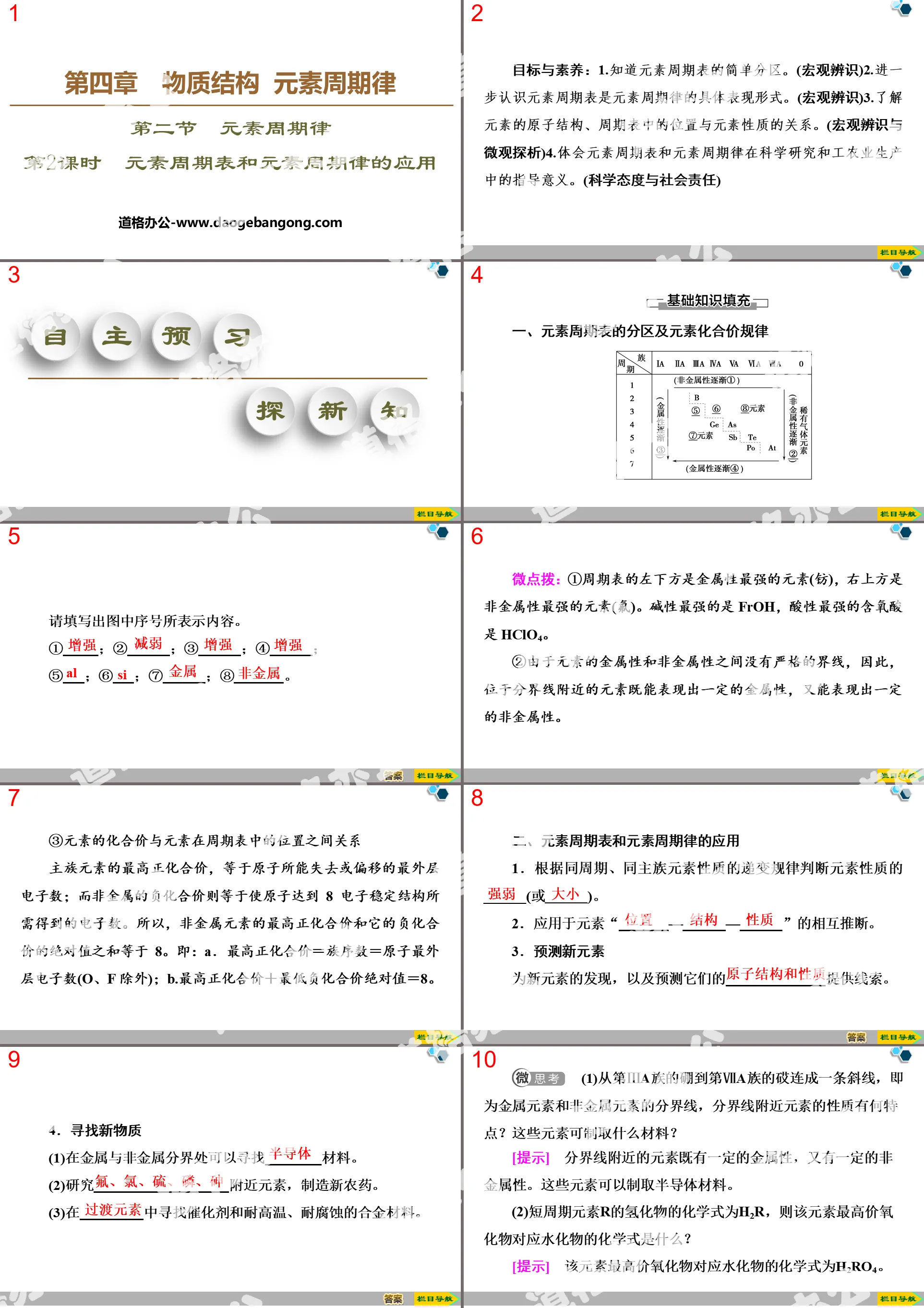
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Classification and Transformation of Matter" PPT is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Classification and Transformation of Matter" PPT, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)

Related reading
For more detailed PPT-related tutorials and font tutorials, you can view:Please click to see








Authoritative PPT Summary
"Classification and Transformation of Matter" PPT
Part One: Simple Classification and Its Application
1. The meaning of classification:
For work and life, reasonable classification of materials can provide convenience for work and life and achieve high efficiency.
For chemical research, rational classification of substances facilitates understanding of the commonalities of various substances and the nature of various reactions.
2. Simple classification method
(1) Cross classification method:
A method of classifying objects by applying several different criteria.
Advantages: Classifying substances from multiple perspectives reflects the various properties of substances and makes up for the shortcomings of a single classification method.
(2) Tree classification method
Tree classification is a multi-level classification method that reclassifies various types of things after classification.
The classification principle of tree classification: substance categories at the same level are generally independent of each other and have no overlap.
Advantages: It reflects the process of gradually deepening the understanding of things.
[Self-examination and mutual evaluation]
Please classify the following substances and fill in the corresponding positions in Figure 2-3 on P25 of the textbook:
Air, C, Cu, Ar, CaO, CO2, HCl, solution, H2SO4, NaOH, sediment, Ba(OH)2, Na2SO4, NaHCO3
【Expansion Migration】
Where can you place the substances NH3 and ethanol (C2H5OH) you have learned in Figure 2-3 on P25 of the textbook?
【Thinking and communication】
In what ways can you classify the following equations?
①2KClO3 ==== 2KCl + 3O2↑
②H2 + CuO === Cu + H2O
③Fe + H2SO4 = FeSO4 + H2↑
④2H2O2 ==== 2H2O + O2↑
⑤3CO+ Fe2O3 ===2Fe + 3CO2
⑥2Ca + O2 = 2CaO
⑦CaO + H2O = Ca(OH)2
⑧H2CO3 + Ca(OH)2 = CaCO3 + 2H2O
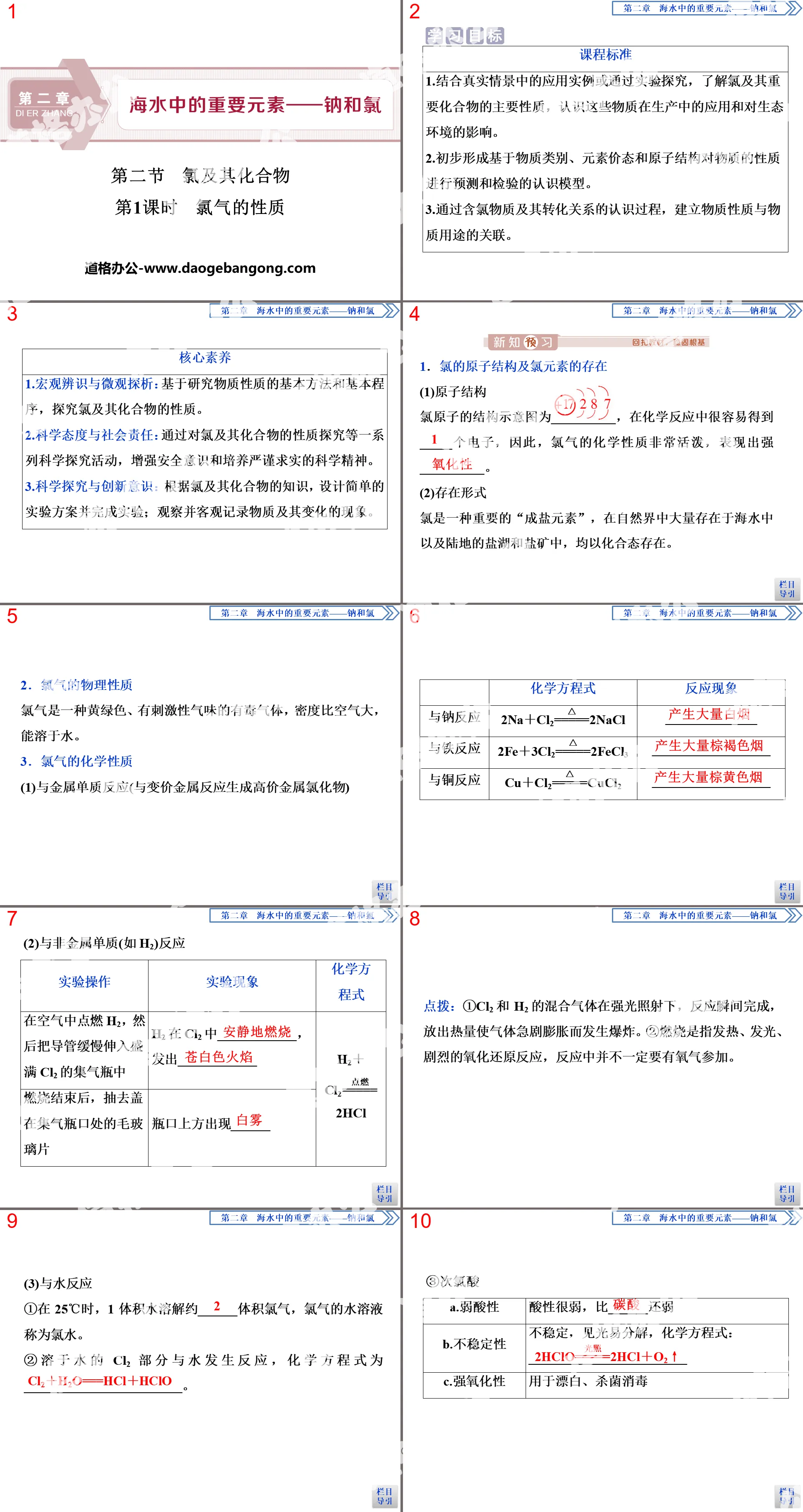
Classification and transformation of substances PPT, Part 2: Discussion and exploration-Classification of chemical reactions
①2KClO3 = 2KCl + 3O2↑
②H2 + CuO = Cu + H2O
③Fe + H2SO4 = FeSO4 + H2↑
④2H2O2 = 2H2O + O2↑
⑤3CO+ Fe2O3 = 2Fe + 3CO2
⑥2Ca + O2 = 2CaO
⑦CaO + H2O = Ca(OH)2
⑧H2CO3 + Ca(OH)2 = CaCO3 ↓ + 2H2O
Classification and transformation of substances PPT, Part 3: Classification of chemical reactions
Thinking Review: Four Basic Response Types:
Classification criteria: According to the categories of reactants and products and the number of substances before and after the reaction
Combination reaction A + B —— C
Decomposition reaction AB - A + B
Displacement reaction A+BC—— B+AC
Metathesis reaction AB + CD —— AD + CB
Expand and extend:
Chemical reactions: ionic reactions, nonionic reactions
Redox reactions
non-redox reaction
exothermic reaction, endothermic reaction
Keywords: PPT courseware for high school chemistry compulsory course 1 from the People's Education Press is available for free download, Classification and Transformation of Substances PPT download, .PPT format;
For more information about the "Classification and Transformation of Substances" PPT courseware, please click on the "Classification and Transformation of Substances" ppt tag.
"Classification and Transformation of Substances" Substances and their Changes PPT (Transformation of Substances in Lesson 2):
"Classification and Transformation of Substances" Substances and their Changes PPT (Transformation of Substances in Lesson 2) Part One Content: Learning Objectives Course Standards 1. Understand the properties of acids, bases, and salts. 2. Recognize that similar substances have similar properties and that various substances can transform into each other under certain conditions..
"Classification and Transformation of Substances" Substances and their Changes PPT (Classification of Substances in Lesson 1):
"Classification and Transformation of Substances" Substances and their Changes PPT (Classification of Substances in Lesson 1) Part One Content: Learning Objectives Course Standards 1. Understand that elements can form different types of substances, and substances can be classified according to their composition and properties. . 2. Understand the points...
"Transformation of Matter" Classification and Transformation of Matter PPT download:
"Transformation of Matter" Classification and Transformation of Matter PPT Download Part One Content: Literacy Objective 1. By reviewing the reactions between acids, bases, salts and different types of substances, summarize the main chemical properties and reaction types of acids, bases, and salts, and cultivate Changing concepts and evidential reasoning abilities..