| Category | Format | Size |
|---|---|---|
| Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"How to Make Oxygen" Mystery of Air PPT Courseware 2
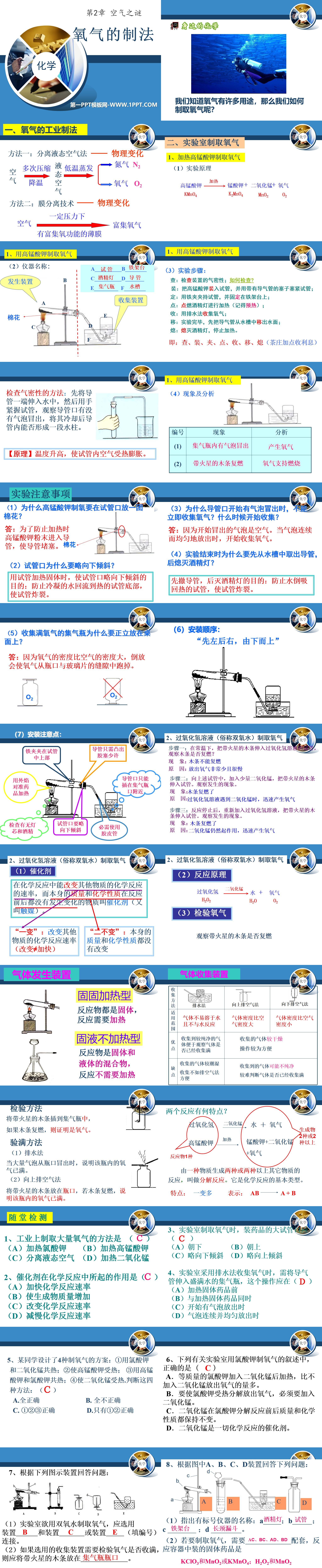
1. Industrial production method of oxygen
Method 1: Separating liquid air─physical changes
Method 2: Membrane separation technology─physical changes
2. Laboratory preparation of oxygen
1. Heating potassium permanganate to produce oxygen
(1) Experimental principle
Potassium permanganate→potassium manganate+manganese dioxide+oxygen
(2) Instrument name:
A test tube B iron stand
C alcohol lamp D catheter
EGas collecting bottle FTank
(3) Experimental steps:
Check: Check the air tightness of the device; how to check?
Installation: Put potassium permanganate into the test tube, and plug the test tube tightly with a stopper with an air guide tube;
Fixation: Clamp the test tube with iron clamps and fix it on the iron stand;
Point: Light the alcohol lamp for heating (remember to preheat);
Collect: Use drainage method to collect oxygen;
Move: After the experiment is completed, first move the air tube from the water tank out of the water;
Off: Extinguish the alcohol lamp and stop heating.
That is: check, install, clip, point, close, move, and put out (the tea house charges interest for adding points)
Experimental Notes
(1) Why does potassium permanganate need to put a ball of cotton at the mouth of the test tube to produce oxygen?
Answer: In order to prevent potassium permanganate powder from entering the conduit during heating and causing the conduit to be blocked.
(2) Why should the mouth of the test tube be slightly inclined downward?
When using a test tube to heat a solid, the purpose of tilting the test tube mouth slightly downward is to prevent the condensed water from flowing back to the bottom of the hot test tube and causing the test tube to burst.
(3) Why can't oxygen be collected immediately when bubbles start to emerge from the catheter port? When does collection start?
Answer: Because the bubbles that start to appear are air. When the bubbles are released continuously and evenly, oxygen collection begins.
(4) At the end of the experiment, why should we first remove the catheter from the water tank and then extinguish the alcohol lamp?
The purpose of removing the catheter first and then extinguishing the alcohol lamp is to prevent water from sucking back into the heated test tube and causing the test tube to burst.
(5) Why should a gas collecting bottle filled with oxygen be placed upright on the table?
Answer: Because the density of oxygen is greater than that of air, placing it upside down will cause the oxygen to escape from the gap between the bottle mouth and the glass piece.
2. Produce oxygen from hydrogen peroxide solution (commonly known as hydrogen peroxide)
Step 1: At normal temperature, put the wooden stick with sparks into the test tube of hydrogen peroxide solution and observe whether the wooden stick re-ignites?
Phenomenon: Wooden strips cannot re-ignite
Reason: The release of oxygen is very little and very slow
Step 2: Add a small amount of manganese dioxide to the above test tube, and insert the wooden stick with sparks into the test tube. Observe what happens.
Phenomenon: The wood sticks rekindled
Reason: When hydrogen peroxide solution encounters manganese dioxide, it quickly generates oxygen
Step 3: After the reaction stops, re-add the hydrogen peroxide solution and insert the wooden stick with sparks into the test tube. Observe what happens.
Phenomenon: The wood sticks rekindled
Reason: Manganese dioxide still works and rapidly produces oxygen
On-site testing
1. The industrial method for producing large amounts of oxygen is ( )
(A) Heating potassium chlorate (B) Heating potassium permanganate
(C) Separating liquid air (D) Heating manganese dioxide
2. The role of catalysts in chemical reactions is ( )
(A) Speed up the rate of chemical reactions
(B) Increase the mass of the product
(C) Change the rate of chemical reactions
(D) Slow down the rate of chemical reactions
3. When preparing oxygen in the laboratory, the mouth of the large test tube containing drugs should be ( )
(A) Down (B) Up
(C) Slightly inclined downward (D) Slightly inclined upward
4. When the laboratory uses the drainage method to collect oxygen, the air guide tube needs to be extended into the gas collecting bottle filled with water. This operation should be done in ( )
(A) Before heating solid pharmaceuticals
(B) Simultaneously with heating solid drugs
(C) When bubbles begin to release
(D) When bubbles are released continuously and evenly
Keywords: Mystery of Air teaching courseware, Oxygen production method teaching courseware, Beijing curriculum reform version ninth grade chemistry PPT courseware download, Ninth grade chemistry slide courseware download, Air Mystery PPT courseware download, Oxygen production method PPT courseware download ,.PPT format;
For more information about the PPT courseware "The Mystery of Air: How to Make Oxygen", please click on the "Mystery of Air ppt: How to Make Oxygen ppt" tag.
"How to Make Oxygen" Mystery of Air PPT Courseware 3:
"How to Make Oxygen" Mystery of Air PPT Courseware 3 Learning Objectives 1. Understand the methods of industrial production of oxygen; understand the main methods and principles of producing oxygen in the laboratory, and initially understand the methods of producing new substances through chemical experiments; 2 , Understand catalysts, catalysis and...
"How to Make Oxygen" PPT Courseware on the Mystery of Air:
"How to Make Oxygen" Mystery of Air PPT courseware 1. Laboratory preparation method Raw materials: Potassium chlorate (white solid) or potassium permanganate (purple black solid) Method: Heating under certain conditions 1. Heating potassium chlorate to produce oxygen, often Add the catalyst manganese dioxide. Experiment ..
File Info
Update Time: 2024-07-11
This template belongs to Chemistry courseware Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1 industry PPT template
"How to Make Oxygen" Mystery of Air PPT Courseware 2 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "How to Make Oxygen" Mystery of Air PPT Courseware 2 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"How to Make Oxygen" Mystery of Air PPT Courseware 2, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview