People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"Atoms" PPT courseware of particles constituting matter
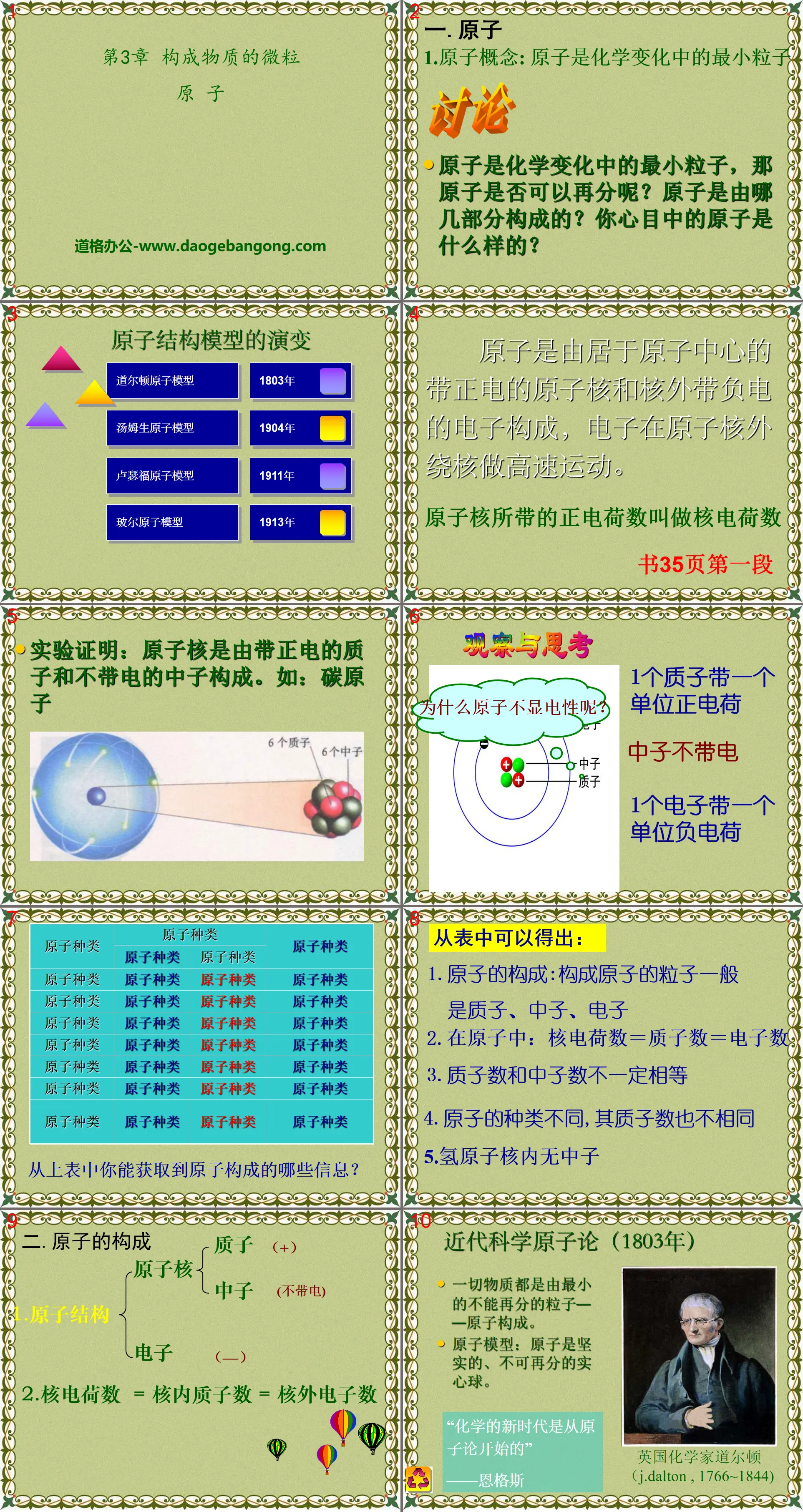
1. Atom
1. Atomic concept: Atom is the smallest particle in chemical changes
Atoms are the smallest particles in chemical changes. Can atoms be subdivided? What parts are atoms made of? What do atoms look like in your mind?
Atoms are composed of a positively charged nucleus at the center of the atom and negatively charged electrons outside the nucleus. The electrons move around the nucleus at high speed.
The number of positive charges carried by the nucleus is called the nuclear charge
Observe and think
1 proton carries one unit of positive charge
Neutrons are uncharged
1 electron carries one unit of negative charge
It can be concluded from the table:
1. The composition of atoms: The particles that make up atoms are generally protons, neutrons, and electrons
2. In an atom: nuclear charge = number of protons = number of electrons
3. The number of protons and the number of neutrons are not necessarily equal
4. Different types of atoms have different numbers of protons.
5. There are no neutrons in the hydrogen nucleus
2. The composition of atoms
1.Atomic structure
Nucleus
Proton (+)
Neutron (uncharged)
electronic(-)
2. Nuclear charge = Number of protons inside the nucleus = Number of electrons outside the nucleus
Alpha particles: The deflection direction in the magnetic field is opposite to the direction of electrons, indicating that the alpha particles are charged. After calculation, we know that the charge of an alpha particle is twice that of an electron. The mass of an alpha particle is 1/49 of the mass of a gold atom, and its mass is 7,000 times that of an electron.
Gold foil is solid, with 2,000 gold atoms tightly packed.
Most alpha particles do not change their original direction of motion;
A small number of alpha particles change their original path of motion;
A very small number of alpha particles are bounced back
Please analyze the structure of atoms based on the above phenomena.
There is a large space inside the atom.
There is a positively charged nucleus inside the atom.
The mass of gold nuclei is much greater than that of alpha particles
atom
Characteristics of atoms
①Atoms are small, have mass and volume
②Atoms are always moving
③There are gaps between atoms
④The atomic properties of the same substance are the same, and the atomic properties of different substances are different.
The most essential difference between different types of atoms
The number of protons in the nucleus is different
How much is the mass of an atom?
The mass of a carbon atom is:
0.00000000000000000000000001993kg
That is, 1.993×10-26 kg. The mass of an oxygen atom is:
0.00000000000000000000000002657kg
That is, 2.657×10-26 kg. The mass of an iron atom is:
0.00000000000000000000000009288kg
That is 9.288×10-26 kg
3. Relative atomic mass
The mass of an atom is so small that it is inconvenient to write and remember.
1. International regulations: 1/12 of the mass of a carbon atom is used as the standard, and the mass of other atoms is compared with it.
2. Calculation of relative atomic mass approximate values:
According to experimental determination:
The mass of an electron is very small, 1/1836 of the mass of a proton
Therefore, the mass of an atom is mainly concentrated in the nucleus
Relative atomic mass ≈ number of protons + number of neutrons
Keywords: Particles constituting matter teaching courseware, atomic teaching courseware, Beijing curriculum reform version ninth grade chemistry PPT courseware download, ninth grade chemistry slide courseware download, Particles constituting matter PPT courseware download, atoms PPT courseware download, .PPT format ;
For more information about the PPT courseware "Particle Atoms Constituting Matter", please click on the PPT Atom PPT tag that constitutes matter.
"Atomic Structure and Periodic Table of Elements" Periodic Law of Material Structure PPT (Lesson 2 Atomic Structure and Properties of Elements):
"Atomic Structure and Periodic Table of Elements" Periodic Law of Material Structure Elements PPT (Lesson 2 Atomic Structure and Properties of Elements) Part One Content: Learning Objectives Course Standards 1. Understand the properties of alkali metal elements and halogen elements and their placement in the periodic table of elements relationship between the central position. ..
"Atomic Structure and Periodic Table of Elements" Periodic Law of Elements in Material Structure PPT (Lesson 1 Atomic Structure and Periodic Table of Elements Nuclides):
"Atomic Structure and Periodic Table of Elements" Periodic Law of Material Structure Elements PPT (Lesson 1 Atomic Structure Periodic Table Nuclides) Part One Content: Learning Objectives Course Standards 1. Understand the electron arrangement outside the nucleus. 2. Know the structure of the periodic table of elements. 3. Know..
"Atomic Structure and Properties of Elements" Atomic Structure and Periodic Table of Elements PPT Download:
"Atomic Structure and Properties of Elements" Atomic Structure and Periodic Table PPT Download Part One: Literacy Objectives 1. Know the atomic structure and characteristics of alkali metal elements and halogen elements through the atomic structure and atomic radius information in the textbook tables. 2. Through teaching materials..
File Info
Update Time: 2024-11-19
This template belongs to Chemistry courseware Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Atoms" PPT courseware of particles constituting matter Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Atoms" PPT courseware of particles constituting matter is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Atoms" PPT courseware of particles constituting matter, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview