People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"Changes of Water" The most common liquid - water PPT courseware
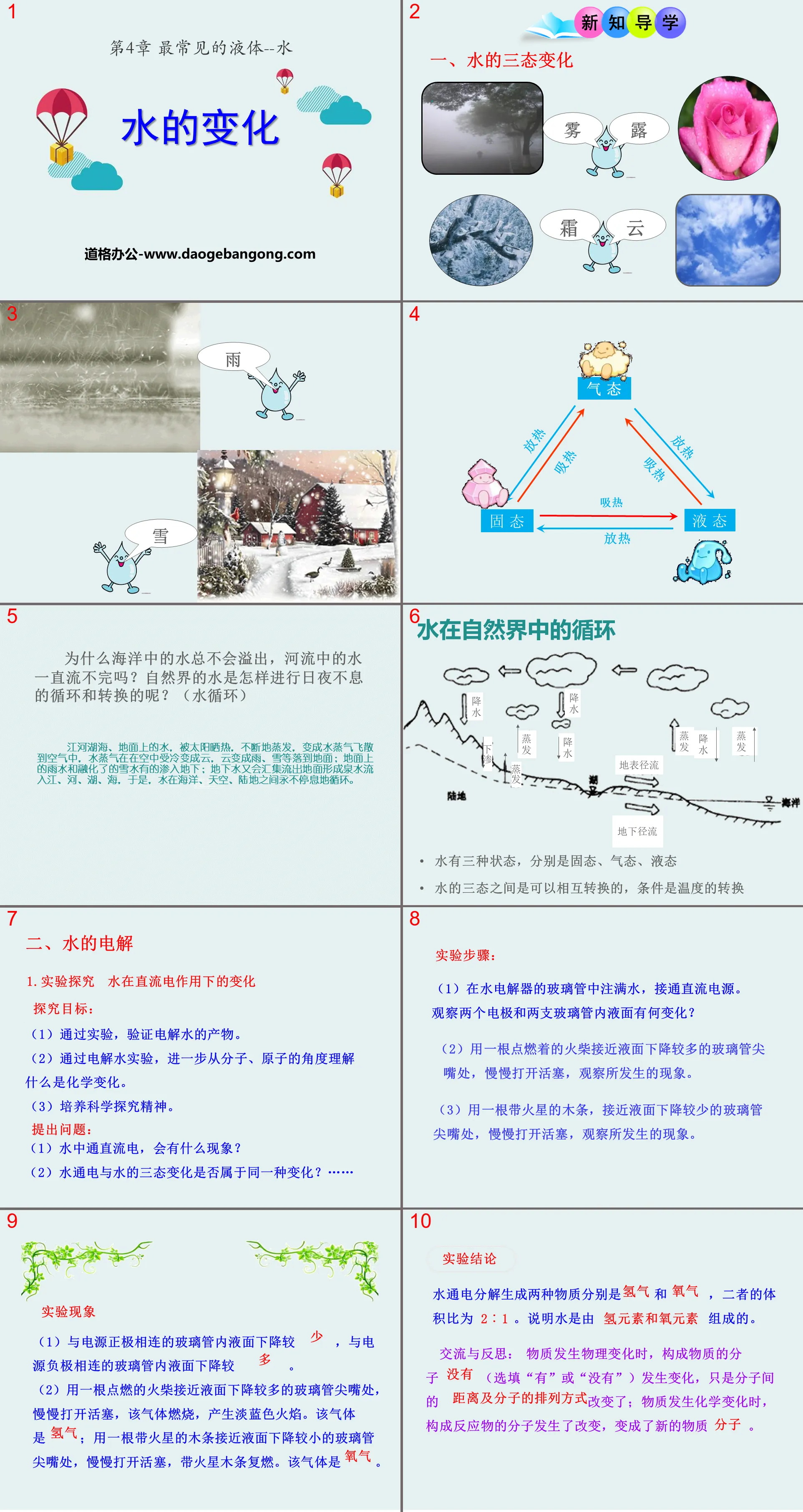
1. Three state changes of water
Why does the water in the ocean never overflow and the water in the river never stop flowing? How does water in nature cycle and transform day and night? (water cycle)
Water in rivers, lakes, seas, and on the ground is heated by the sun and constantly evaporates, turning into water vapor and flying into the air. The water vapor is cooled in the air and turns into clouds, and the clouds turn into rain, snow, etc. and fall to the ground; the ground Some of the rainwater and melted snow water seep into the ground; the groundwater will collect and flow out of the ground to form spring water and flow into rivers, rivers, lakes, and seas. As a result, water circulates endlessly among the ocean, sky, and land.
water cycle in nature
Water has three states: solid, gas, and liquid
The three states of water can be converted into each other, the condition is the conversion of temperature
2. Electrolysis of Water
1. Experimental study on the changes of water under the action of direct current
Research objectives:
(1) Through experiments, verify the products of electrolyzed water.
(2) Through the electrolysis of water experiment, we can further understand what chemical changes are from the perspective of molecules and atoms.
(3) Cultivate the spirit of scientific inquiry.
Ask a question:
(1) What will happen if direct current is passed through the water?
(2) Are the electricity flowing through water and the three-state changes of water the same kind of change? …
Experimental steps:
(1) Fill the glass tube of the water electrolyzer with water and connect the DC power supply. Observe the changes in the liquid level in the two electrodes and the two glass tubes?
(2) Use a lit match to approach the tip of the glass tube where the liquid level has dropped more, slowly open the piston, and observe what happens.
(3) Use a wooden stick with sparks, close to the tip of the glass tube where the liquid level drops less, slowly open the piston, and observe what happens.
Schematic diagram of the decomposition of water molecules:
According to the schematic diagram of the decomposition of water molecules, please describe the information you obtained from both macro and micro aspects (3 to 4 items)
micro aspects
(1) Water is composed of water molecules;
(2) Water molecules are composed of hydrogen atoms and oxygen atoms;
(3) Oxygen molecules are composed of oxygen atoms; hydrogen molecules are composed of hydrogen atoms;
(4) In chemical changes, molecules can be divided into atoms, but atoms cannot be divided further;
(5) Atoms are the smallest particles in chemical changes.
macro aspect
(1) Water is composed of hydrogen and oxygen elements;
(2) Oxygen is composed of oxygen element;
(3) Hydrogen gas is composed of hydrogen element...
3. Hydrogen:
Hydrogen is a colorless, odorless, poorly soluble gas that produces a light blue flame when burned in the air...
Hydrogen mixed with a certain amount of air or oxygen will explode when exposed to an open flame. Therefore, the purity of hydrogen must be checked before igniting it.
combustion of hydrogen
Hydrogen can be ignited. This is a chemical reaction between hydrogen and oxygen in the air. Does the reaction produce water?
Phenomenon: Pure hydrogen burns in the air, producing a light blue flame, and water droplets condense on the wall of the beaker. If you touch the beaker with your hand and it feels hot, it means that the hydrogen gas reacted with the oxygen in the air to form water and release heat.
Literal expression for reaction:
hydrogen + oxygen → water
H2 O2 H2O
Hydrogen energy
(1) Hydrogen energy is an extremely ideal energy source with broad development prospects. It has the following advantages:
①Raw materials are not restricted (wide sources)
② Combustion releases a lot of heat (high calorific value)
③The product does not pollute the environment (no pollution) (the biggest advantage)
Why hydrogen energy is not widely used
① Flammable and explosive
②Difficult to liquefy
③ Storage and transportation are unsafe and inconvenient
④ Preparing hydrogen consumes a lot of electricity and costs a lot.
Keywords: The most common liquid water teaching courseware, the change of water teaching courseware, the Beijing curriculum reform version of the ninth grade chemistry PPT courseware download, the ninth grade chemistry slide courseware download, the most common liquid water PPT courseware download, the change of water PPT courseware Courseware download, .PPT format;
For more information about the PPT courseware "Changes in Water: The Most Common Liquid Water", please click on the "Changes in Water ppt: The Most Common Liquid Water" ppt tag.
"Three State Changes of Water" PPT download of changes in temperature and water:
"Three State Changes of Water" Temperature and water changes PPT download Part 1: Introduction of new knowledge Do you still remember ice? Is it a solid, liquid, or gas? What about water? Now that we have solids and liquids, what is missing? Today we will learn about the three states of water...
"Three State Changes of Water" Changes in Temperature and Water PPT:
"Three State Changes of Water" Temperature and Water Changes PPT Part One: Various Forms of Water Water has various forms in nature, sometimes liquid, sometimes solid, and sometimes gaseous. Based on the phenomena we have observed and our existing life experiences, let’s talk about it...
"Water and Water Vapor" Changes in Temperature and Water PPT:
"Water and Water Vapor" Temperature and water changes PPT Part 1: Introduction of new knowledge Where does the water on the wall of the cup come from? After it rains, there will be some puddles on the ground. The weather cleared up and the water in the puddles dried up quickly. Where did the water in the puddle go? Water seeps into...
File Info
Update Time: 2024-11-21
This template belongs to Chemistry courseware Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Changes of Water" The most common liquid - water PPT courseware Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Changes of Water" The most common liquid - water PPT courseware is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Changes of Water" The most common liquid - water PPT courseware, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview