People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
Hunan Education Edition Ninth Grade Chemistry Volume 1
Cantonese Education Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 2
People's Education Press High School Chemistry Compulsory Course 2
Lu Ke Edition High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2

| Category | Format | Size |
|---|---|---|
| Hunan Education Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
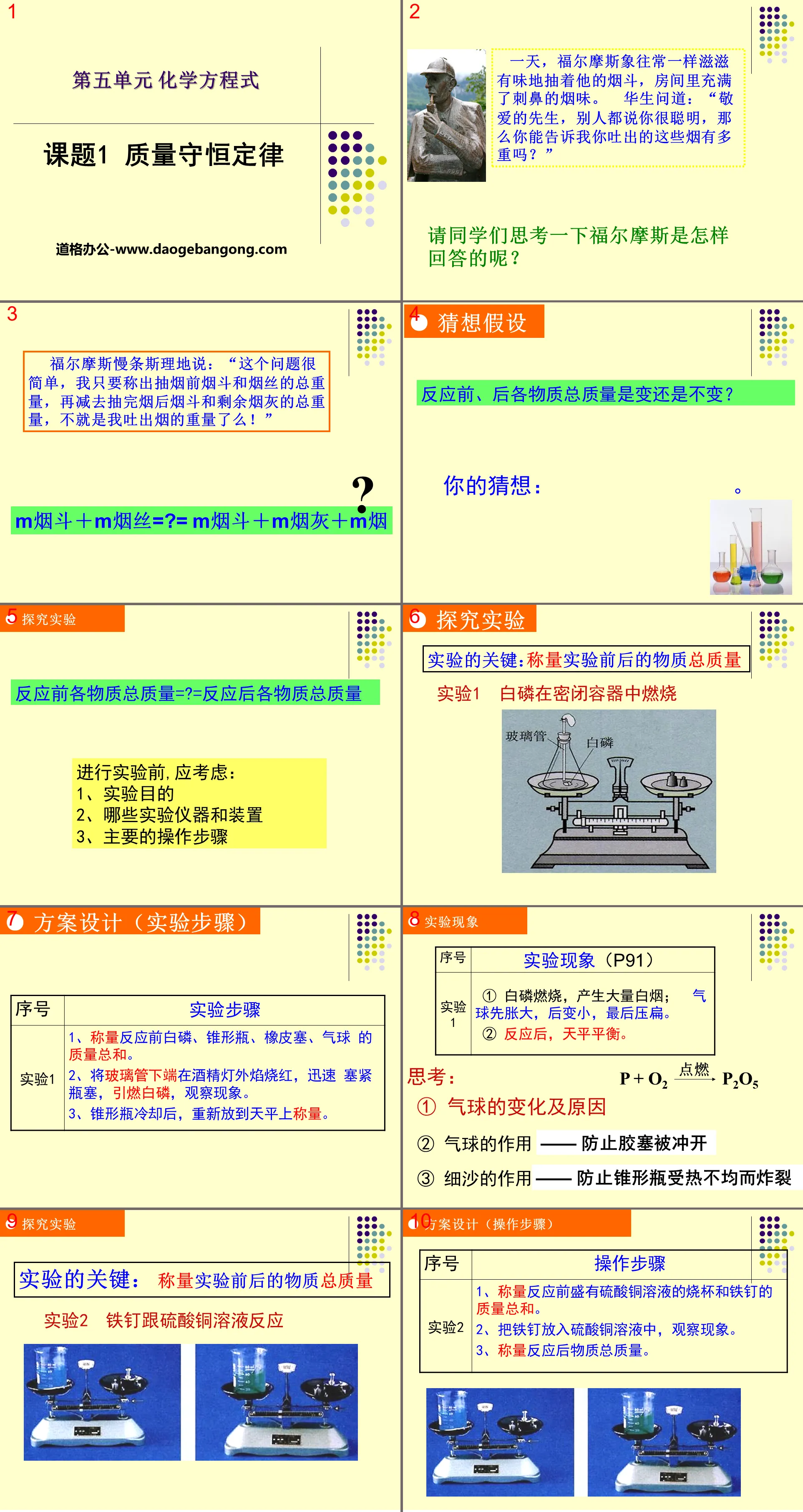
"Law of Conservation of Mass" Chemical Changes and Their Representation PPT Courseware 2
One day, Holmes was smoking his pipe as usual, and the room was filled with the pungent smell of smoke. Watson asked: "Dear sir, others say you are very smart, so can you tell me how heavy the smoke you exhale weighs?"
Ask the students to think about how Holmes answered?
Holmes said slowly: "This question is very simple. I only need to weigh the total weight of the pipe and tobacco before smoking, and then subtract the total weight of the pipe and remaining ash after smoking. Isn't it the weight of the smoke I exhale!"
m pipe + m cut tobacco =?= m pipe + m ash + m cigarette
Exploration experiment
The total mass of each substance before the reaction =? = the total mass of each substance after the reaction
Before conducting experiments, you should consider:
1. Experimental purpose
2. What experimental instruments and devices
3. Main operating steps
Solution design (experimental steps)
Experiment 1
Experimental steps
1. Weigh the total mass of white phosphorus, Erlenmeyer flask, rubber stopper, and balloon before reaction.
2. Heat the lower end of the glass tube red in the flame outside the alcohol lamp, quickly plug the bottle stopper, ignite the white phosphorus, and observe the phenomenon.
3. After the Erlenmeyer flask has cooled, place it on the balance again and weigh it.
Experimental phenomena (P91)
① White phosphorus burns and produces a large amount of white smoke; the balloon first inflates, then becomes smaller, and finally collapses.
② After the reaction, balance the balance.
think:
① Changes and causes of balloons
② The role of the balloon - to prevent the rubber stopper from being blown open
③ The role of fine sand - to prevent the Erlenmeyer flask from exploding due to uneven heating
get rules
1. Law of conservation of mass (page 91 of the textbook)
Numerous experiments have proven:
The sum of the masses of each substance participating in a chemical reaction is equal to the sum of the masses of each substance produced after the reaction.
P + O2 → P2O5
Fe + CuSO4 → Cu + FeSO4
1. The law of conservation of mass applies only to chemical changes.
2. "Mass" must be the mass of the substance that actually participates in the chemical reaction, rather than the simple sum of the masses of each substance.
3. Sum: The gas "gas product CO2 H2 O2" in the reactants or products cannot be ignored.
4. It is "mass conservation", not volume conservation.
practise:
1. In the chemical reaction A + B = C,
4g 12g
Then the mass of B participating in the reaction is ( )
A.6 grams B.8 grams C.10 grams D.16 grams
2. The phenomenon that cannot be explained by the law of conservation of mass is ( )
A. Dry wet clothes in the sun
B. The iron wire burns and its solid mass increases
C. After potassium permanganate is heated, the solid mass decreases
D. After the candle burns, it becomes shorter and shorter and eventually disappears
Keywords: teaching courseware on the law of conservation of mass, teaching courseware on chemical changes and their representations, download the chemistry PPT courseware for the first volume of the ninth grade of the Hunan Education Edition, download the chemistry slide courseware for the ninth grade, download the PPT courseware on the law of conservation of mass, chemical changes and their representations PPT courseware Download, .PPT format;
For more information about the "Law of Conservation of Mass Chemical Changes and Their Representations" PPT courseware, please click on the PPT Label of "Law of Conservation of Mass Chemical Changes and Their Representations" PPT.
"Law of Conservation of Mass" Chemical Equations PPT Courseware (Lesson 2):
"Law of Conservation of Mass" Chemical Equations PPT Courseware (Lesson 2), 12 pages in total. Introduction of new lesson: Which one is better to express chemical reactions in various ways? Type 3 is the best if it requires simplicity and generality. Like formula ③, a chemical formula is used to express a chemical reaction..
"Law of Conservation of Mass" Chemical Equations PPT Courseware (Lesson 1):
"Law of Conservation of Mass" Chemical Equations PPT Courseware (Lesson 1), 15 pages in total. Introduction to New Lesson We already know that the result of a chemical reaction is the conversion of reactants into products. So, how do the masses of reactants and products change during a chemical reaction?
"Law of Conservation of Mass" Chemical Equation PPT (Lesson 2):
"Law of Conservation of Mass" Chemical Equations PPT (Lesson 2), 16 pages in total. Learning Objectives 1. Through the chemical equation of carbon combustion, be able to obtain and state the relevant reaction information from the specific chemical equation, and summarize the meaning of the chemical equation. 2. Through carbon..
File Info
Update Time: 2024-10-03
This template belongs to Chemistry courseware Hunan Education Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Law of Conservation of Mass" Chemical Changes and Their Representation PPT Courseware 2 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Law of Conservation of Mass" Chemical Changes and Their Representation PPT Courseware 2 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Law of Conservation of Mass" Chemical Changes and Their Representation PPT Courseware 2, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview