| Category | Format | Size |
|---|---|---|
| Cantonese Education Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"Combustion Conditions and Fire Extinguishing Principles" Life-Sustaining Gas - Oxygen PPT Courseware 2
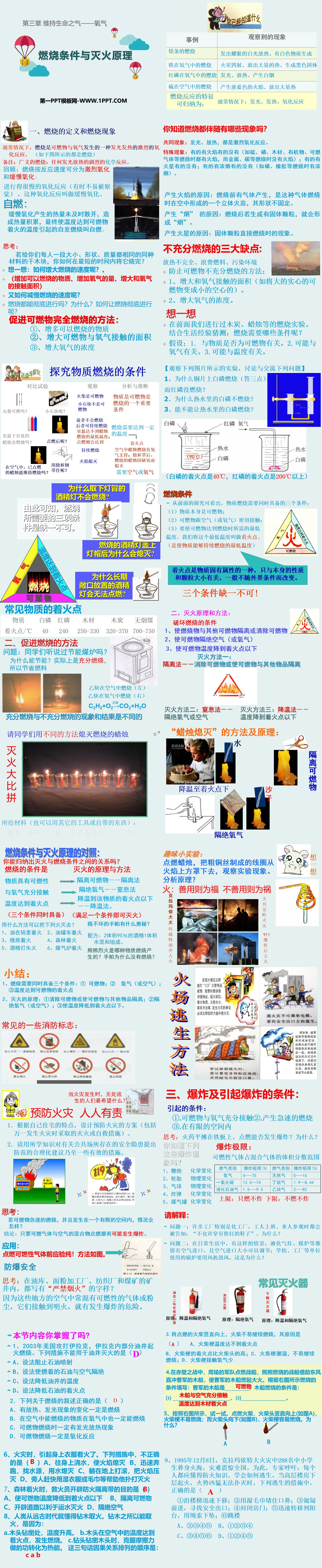
1. Definition of combustion and combustion phenomena
Normally, combustion is an intense oxidation reaction between combustibles and oxygen that produces heat and light. (As shown in the picture below, everything is burning)
Note: Combustion in a broad sense: any violent chemical reaction that emits light and heat.
Review: Combustion can be divided into intense oxidation and slow oxidation according to reaction speed:
An oxidation reaction that proceeds very slowly (sometimes not easily noticeable) is called slow oxidation.
Spontaneous combustion:
The heat generated by slow oxidation is not dissipated in time, causing heat accumulation, and eventually the temperature reaches the temperature at which combustibles ignite, causing spontaneous combustion, which is called spontaneous combustion.
Do you know what phenomena are associated with combustion?
Common phenomena: luminescence and heat release, both of which are intense oxidation reactions.
Special phenomenon: some have flames and some do not (for example, sulfur, phosphorus, wood, organic matter, combustible gases, etc. all have flames when burning, while metals, carbon, etc. do not have flames when burning); some have sparks and some do not; some There is thick smoke and some there is not (for example, there is thick smoke when burning phosphorus, rubber, etc.).
The reason for the flame: gas is produced before combustion. It is a three-dimensional flame formed in the air when the gas burns. Its shape is not fixed.
Causes of "smoke": If solid particles are generated after combustion, "smoke" will be formed.
Cause of Mars: The phenomenon when solid particles are directly burned.
Three major disadvantages of incomplete combustion:
Incomplete heat release, waste of fuel, and environmental pollution
Methods to prevent incomplete combustion of combustibles:
1. Increase the area in contact with oxygen (such as turning large solid combustible materials into small hollow ones).
2. Increase the concentration of oxygen.
think about it
Previously we have conducted burning experiments with charcoal, candles, etc. Guess based on life experience: What conditions are needed for combustion?
Hypothesis: 1. Relevant to whether the substance is combustible. 2. May be related to oxygen. 3. It may be related to temperature.
combustion conditions
From the previous exploration, we can see that three conditions need to be met at the same time for material combustion:
(1) The substance itself is combustible;
(2) Combustibles are in close contact with air (or oxygen);
(3) Make combustible materials reach the minimum required for combustion.
Temperature, we call this minimum temperature the ignition point. (is the lowest temperature at which a substance can continue to burn)
2. Principles and methods of fire extinguishing:
Destroy burning conditions
1. Separate burning materials from other combustibles or remove combustibles
2. Isolate combustibles from air (or oxygen)
3. Reduce the temperature of combustible materials below the ignition point
Fire extinguishing method one:
Isolation method - eliminate flammable materials or isolate flammable materials from other items
Fire extinguishing method two:
Asphyxiation - cutting off oxygen or air
Fire extinguishing method three:
Cooling method - the temperature drops below the ignition point
summary:
1. Combustion requires three conditions at the same time: ① combustibles; ② oxygen (or air); ③ the temperature reaches the ignition point of combustibles
2. Principles of fire extinguishing: ① Remove combustibles or isolate combustibles from other items; ② Isolate oxygen (or air); ③ Reduce the temperature to below the ignition point.
Fire prevention is everyone’s responsibility
1. According to the characteristics of your own home, design a fire prevention plan (including fire extinguishing or self-rescue measures in case of fire).
2. Please use the knowledge you have learned to put forward reasonable suggestions and even some effective measures to prevent potential safety hazards in public places.
3. Explosion and conditions causing explosion:
Conditions causing:
①.Full contact between combustibles and oxygen
②.Produce rapid combustion
③.In a limited space
Thinking: If gunpowder is spread flat on an iron plate, will it explode if ignited? Why?
Do you know the following explosion phenomena?
1. Chemical changes of firecrackers
2. Tire physical changes
3. Balloon physical changes
4. Bomb chemical changes
5. Gas tank chemical changes
please explain:
Question 1: In many factories, especially chemical factories, workers are told when they go to work or when visitors come to visit: "Shoes with iron nails are not allowed." Why?
Question 2: In daily life, there are situations like this: liquefied gas stoves, coal stoves, etc. have air inlets, and the size of the air inlets can be adjusted; boilers used in schools, factories, and other units require fans to blow. why is that?
Have you mastered the content of this section?
1. In 2003, the United States attacked Iraq, and some oil wells in Iraq caught fire. Which of the following measures cannot be used to extinguish oil well fires ( )
A. Try to stop the oil injection
B. Try to isolate the burning oil from the air
C. Try to lower the temperature of the oil well
D. Try to reduce the ignition point of oil
2. Which of the following statements about combustion is correct ( )
A. Changes that produce heat and light must be combustion
B. Substances that can burn in air will also burn in oxygen
C. When combustibles burn, they must emit light and heat.
D. The combustion of combustibles must be an oxidation reaction
3. Hold the lit match vertically upward. The match will not continue to burn easily. The reason is ( )
A. The temperature of the match stem does not reach the ignition point
B. The ignition point of the match stem is higher than that of the match head;
C. The match stem is moist and difficult to continue burning;
D. Match stems have less exposure to oxygen
Keywords: Oxygen, the life-sustaining gas teaching courseware, combustion conditions and fire extinguishing principles teaching courseware, Guangdong Education Edition ninth grade chemistry PPT courseware download, ninth grade chemistry slide courseware download, life-sustaining gas oxygen PPT courseware download, combustion conditions and PPT courseware download on fire extinguishing principles, in .PPT format;
For more information about the PPT courseware "The Combustion Conditions and Fire-Extinguishing Principles of Oxygen, a Life-Sustaining Gas", please click the Combustion Conditions and Fire-Extinguishing Principles of Life-Sustaining Gas Oxygen ppt tag.
"Combustion Conditions and Fire Extinguishing Principles" Life-Sustaining Gas - Oxygen PPT Courseware 3:
"Combustion Conditions and Principles of Fire Extinguishing" Life-Sustaining Gas Oxygen PPT Courseware 3 1. Combustion Conditions What conditions do substances need to meet to burn? Experimental exploration: Analyze wood strips, small pieces of paper, and cotton threads as combustibles; stones, glass rods, and porcelain flakes Not combustible. in conclusion ..
"Combustion Conditions and Fire Extinguishing Principles" Life-Sustaining Gas - Oxygen PPT Courseware:
"Combustion Conditions and Principles of Fire Extinguishing" PPT courseware of oxygen, the life-sustaining gas. The definition of combustion. Normally, combustion is a violent oxidation reaction between combustibles and oxygen that produces heat and light. [Discussion and exchange] 1. Why does the white phosphorus on the copper sheet burn but the red phosphorus does not...
File Info
Update Time: 2024-07-04
This template belongs to Chemistry courseware Cantonese Education Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Combustion Conditions and Fire Extinguishing Principles" Life-Sustaining Gas - Oxygen PPT Courseware 2 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Combustion Conditions and Fire Extinguishing Principles" Life-Sustaining Gas - Oxygen PPT Courseware 2 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Combustion Conditions and Fire Extinguishing Principles" Life-Sustaining Gas - Oxygen PPT Courseware 2, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview