| Category | Format | Size |
|---|---|---|
| Cantonese Education Edition Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"Expressions of the Composition of Matter" The gas that sustains life - Oxygen PPT courseware 3
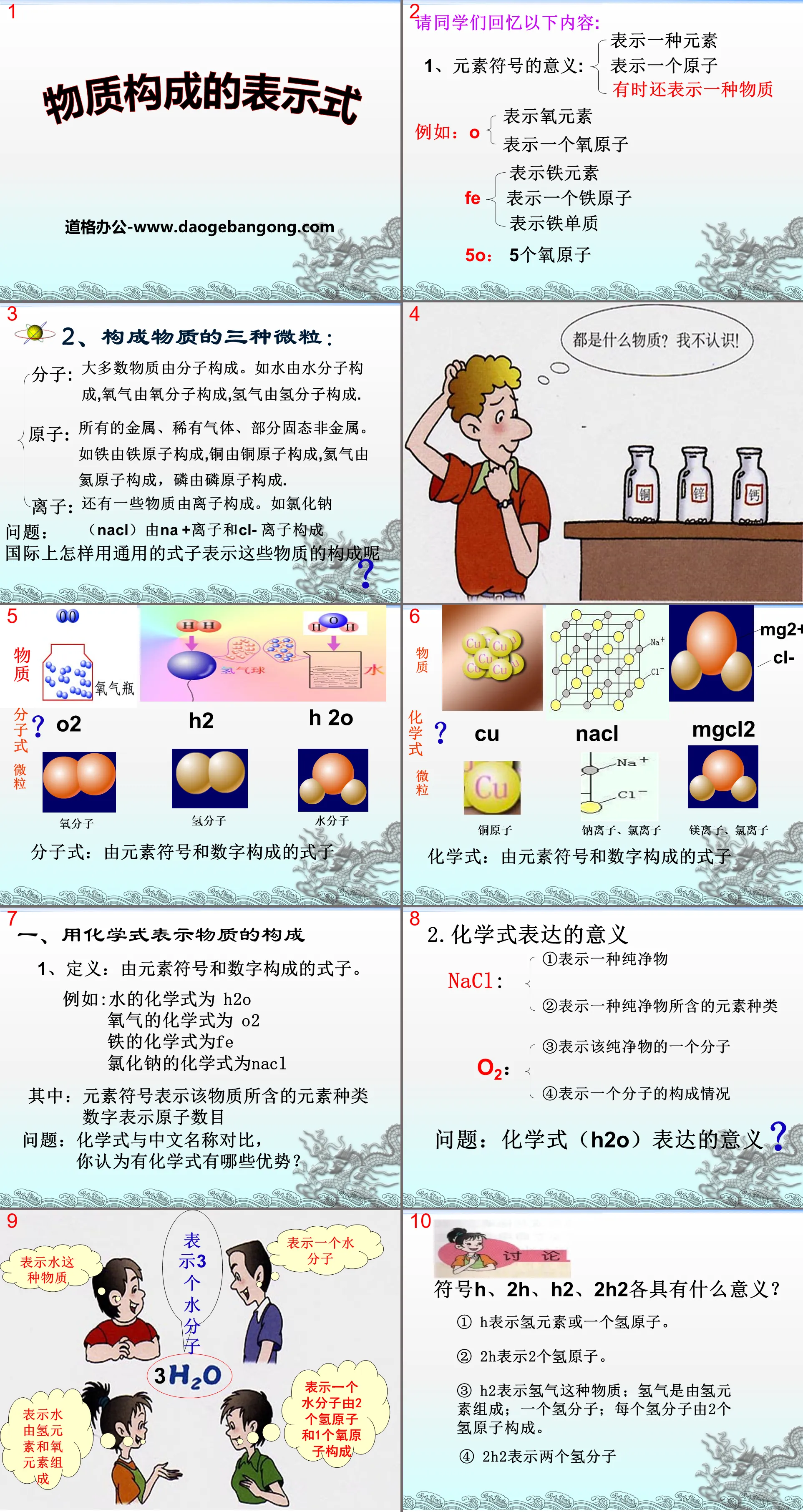
Ask students to recall the following:
1. The meaning of element symbols:
represents an element
represents an atom
Sometimes it also means a substance
2. Three types of particles that make up matter:
Molecules: Most substances are made of molecules. For example, water is made up of water molecules, oxygen is made up of oxygen molecules, and hydrogen is made up of hydrogen molecules.
Atoms: All metals, rare gases, and some solid nonmetals. For example, iron is composed of iron atoms, copper is composed of copper atoms, helium is composed of helium atoms, and phosphorus is composed of phosphorus atoms.
Ions: There are also some substances made of ions. For example, sodium chloride (NaCl) is composed of Na + ions and Cl- ions
Question: How do we use universal formulas to express the composition of these substances in the world?
1. Use chemical formulas to express the composition of substances
1. Definition: A formula composed of element symbols and numbers.
For example: the chemical formula of water is H2O
The chemical formula of oxygen is O2
The chemical formula of iron is Fe
The chemical formula of sodium chloride is NaCl
Among them: the element symbol indicates the type of element contained in the substance and the number indicates the number of atoms.
Question: Comparing the chemical formula with the Chinese name, what do you think are the advantages of having a chemical formula?
2. The meaning of chemical formula expression
NaCl:
①Indicates a pure substance
②Indicates the type of elements contained in a pure substance
O2:
③Represents one molecule of the pure substance
④Indicates the composition of a molecule
Exam question training:
Express or tell the meaning of a symbol using a symbol:
3 potassium atoms______; 2 nitrogen molecules______; 5 nitrogen atoms______;
2 hydrogen atoms ______; 3 carbon dioxide molecules ______.
3H2O____________;
Mg____________________;
Al3+____________________.
Summary: The meaning of the numbers in the chemical formula (R)
(1) a represents the number of particles
(2) b represents the number of atoms.
(3) c means that an ion carries c units of positive (negative) charge
2. Writing of elemental chemical formulas
Represented by the corresponding element symbol
(1)Elements directly composed of atoms
1. Rare gases
Such as: helium (He), neon (Ne), etc.
2. All metals
Such as: magnesium (Mg), iron (Fe), etc.
3. Some solid non-metals
Such as: carbon (C), phosphorus (P), silicon (Si), etc.
(2)Elements composed of molecules
Write the number of atoms in the molecule in the lower right corner of the corresponding element symbol
Valence
1. The compound price is some numerical value, including positive and negative prices.
2. Some elements only show one valence, while some elements can show multiple valences.
3. Atomic groups also show valence.
4. How to express the valence of elements: H2O
5. The valency of elements in a single substance is 0.
6. The algebraic sum of positive and negative valences in a compound is 0.
1.Write chemical formula based on valence
Example 1 It is known that hydrogen has a valence of +1 and oxygen has a valence of -2. Write the chemical formula of this compound of water.
intersection method
1. Write the symbols of the elements in order, with positive valence on the left and negative valence on the right.
2. Mark the valence directly above the element symbol
3. Cross-write the absolute value of the valence to the lower right of the symbol of another element as its number of atoms.
2. Find the valency of elements based on chemical formulas
Principle: The sum of the positive and negative valence algebras in a compound is zero.
How to read chemical formulas (Textbook P91)
A compound composed of two elements: read from right to left as "a certain chemical"; sometimes it is also necessary to read the number of atoms of each element in the chemical formula.
KCl NH4Cl FeCl2 FeCl3 MgO HgS
Potassium chloride Ammonium chloride Ferrous chloride Ferric chloride Magnesium oxide Mercury sulfide
Al2O3 Fe2O3 Na2O MgCl2
Aluminum oxide Iron oxide Sodium oxide Magnesium chloride
CO2 CO SO2 Fe3O4 P2O5 MnO2
Carbon dioxide Carbon monoxide Sulfur dioxide Iron tetroxide Phosphorus pentoxide Manganese dioxide
Compounds containing atomic groups: Read the names of the atomic groups
Containing OH- ions is pronounced "hydroxide"
Example: NaOH reads sodium hydroxide
Containing NO3- ions read "nitric acid"
Example: NaNO3 sodium nitrate
Those containing SO42- ions are read as "sulfuric acid"
Example: CuSO4 copper sulfate
Those containing CO32- ions are read as "carbonic acid"
Example: CaCO3 calcium carbonate
Calculation based on chemical formula
1. Calculate the relative molecular mass of a substance based on its chemical formula
Relative molecular mass: the sum of the relative atomic masses of the atoms in the chemical formula
Exercise: Relative atomic masses: H-1, O-16, C-12, Ca-40, S-32, N-14, Cu-64
Relative molecular mass of CO2 = 12 + 16 x 2 = 44
Relative molecular mass of CaCO3 = 40+12+16x3=100
Relative molecular mass of H2SO4=1x2+32+16x4=98
Relative molecular mass of Cu(OH)2=64+2x(16+1)=98
Relative molecular mass of (NH4) 2SO4=2x(14+1x4)+32+16x4=132
Relative molecular mass of CuSO4 .5H2O=64+32+16x4+5x(1x2+16)=250
2. Calculate the mass ratio of the constituent elements of matter
The chemical formula clearly expresses the elemental composition of the substance and the atomic number ratio of the elements in the compound. Therefore, the mass ratio of the elements in the compound can be calculated through the chemical formula.
(2011 High School Entrance Examination) 5. (This question has 2 questions, totaling 12 points)
twenty four. (5 points) The media recently reported that some instant noodles on the market are contaminated by plasticizers. Long-term exposure to plasticizers can cause damage to the blood system and reproductive system. Among them, the molecular formula of plasticizer (DMP) is C10H10O4. beg:
(1) The relative molecular mass of DMP is _______;
(2) The mass ratio of the three elements C, H, and O in the DMP molecule is ____________;
(3) The mass fraction of oxygen element in DMP molecules is (the result is accurate to 0.01) ________.
[2012 High School Entrance Examination] 5. (This question has 2 questions, totaling 12 points)
twenty four. (5 points) In 2011, Chinese female scientist Tu Youyou discovered artemisinin (chemical formula of artemisinin: C15H22O5), which is a drug used to treat malaria and won the Lasker Award from the United States.
(1) Artemisinin molecules are composed of ______ elements.
(2) An artemisinin molecule contains ______ atoms.
(3) The mass ratio of the three elements C, H, and O in the artemisinin molecule is ____________.
power enhanced
1. The mass ratio of Fe2O3 and Fe3O4 containing the same mass of iron element is ( )
A, 3:2 B, 3:1 C, 30:29 D, 29:42
2. The ratio of the number of oxygen atoms contained in SO2 and SO3 molecules with equal masses is ( )
A, 1:1 B, 2:3 C, 5:6 D, 6:5
3. The mass ratio of SO2 and SO3 containing equal numbers of atoms is ( )
A, 3:4 B, 4:5 C, 15:16 D, 16:15
Keywords: teaching courseware of expressions of the composition of matter, teaching courseware of oxygen, the gas that sustains life, Guangdong Education Edition ninth grade chemistry PPT courseware download, ninth grade chemistry slide courseware download, expression of the composition of matter PPT courseware download, life-sustaining PPT courseware Qi Oxygen PPT courseware download, .PPT format;
For more information about the PPT courseware "Expressions of the substance composition of oxygen, the life-sustaining gas", please click the Expression of the substance composition of oxygen, the life-sustaining gas ppt tag.
"Expressions of the Composition of Matter" Life-Sustaining Gas - Oxygen PPT Courseware 2:
"Expression of the Composition of Matter" Oxygen, the life-sustaining gas PPT courseware 2 1. Chemical formula 1. Definition: A formula that expresses the composition of matter using a combination of element symbols and numbers. For example: the chemical formula of water is, the chemical formula of oxygen is, the chemical formula of carbon dioxide is ① Any substance...
"Expressions of the Composition of Matter" Life-Sustaining Gas - Oxygen PPT Courseware:
"Expression of the composition of matter" PPT courseware of oxygen, the life-sustaining gas. How to use formulas to express the composition of matter. Internationally, the formula that uses a combination of element symbols and numbers to express the composition of matter is called a chemical formula. Water: H2O Hydrogen peroxide: H2O2 Substances composed of molecules...
File Info
Update Time: 2024-07-11
This template belongs to Chemistry courseware Cantonese Education Edition Ninth Grade Chemistry Volume 1 industry PPT template
"Expressions of the Composition of Matter" The gas that sustains life - Oxygen PPT courseware 3 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Expressions of the Composition of Matter" The gas that sustains life - Oxygen PPT courseware 3 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Expressions of the Composition of Matter" The gas that sustains life - Oxygen PPT courseware 3, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview