| Category | Format | Size |
|---|---|---|
| People's Education Press Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"Structure of Atom" The Mystery of Material Composition PPT Courseware 3
Knowledge review
In the experiment of decomposing hydrogen peroxide to produce oxygen, hydrogen peroxide ____ breaks into hydrogen ____ and oxygen ____. The literal expression of this reaction is: ______________. It can be seen that molecules are very small, but they can be ____ in chemical reactions, and ____ cannot be subdivided in chemical changes, so ____________________ is the smallest particle in chemical changes.
(Tip: fill in “molecule” or “atom”)
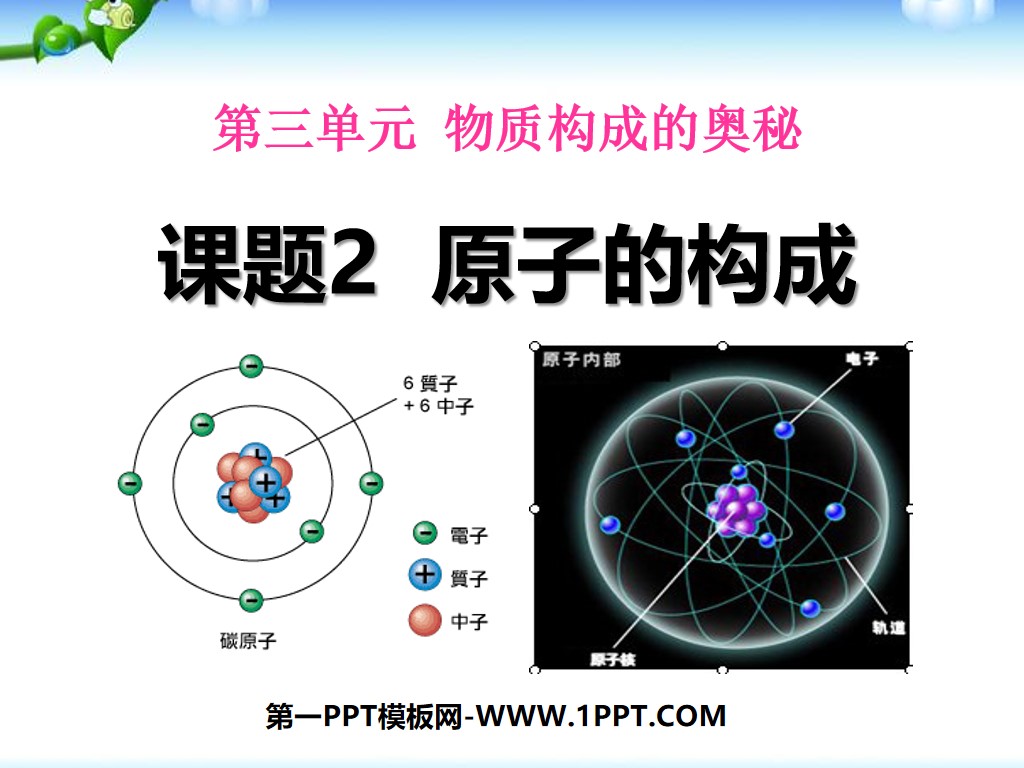
1. The composition of atoms
1. Structure of atoms
Nucleus
Proton (positively charged)
Neutron (uncharged)
Electrons (negatively charged)
Observe the table (Table 3-1) and think about the following questions
1. What are the differences between different atoms?
The number of protons in an atom is different, and the types of atoms are also different.
2. Are all atoms composed of protons, neutrons, and electrons?
Not all atoms have neutrons. such as hydrogen atoms
3. What is the quantitative relationship between the number of protons and the number of neutrons?
The number of protons is not necessarily equal to the number of neutrons.
4. What is the relationship between the number of protons, the number of electrons, the nuclear charge, and the atomic number?
Number of protons = number of electrons outside the nucleus = number of nuclear charges = atomic number
1. Atoms are composed of a nucleus with _____ in the center of the atom and electrons with _____ outside the nucleus. The nucleus is composed of _____ and _____. Because they carry _____ charges and electrical properties ____, the entire atom ____ (fill in the electrical property or not).
2. The smallest particle that can maintain the chemical properties of matter is ____; the smallest particle in chemical changes is _____; the positively charged particles in atoms are _ _________; the non-electric particles are ____, ____, and ____.
3. According to reports, scientists discovered a new element in December 1994. There are 111 protons and 161 neutrons in its atomic nucleus. The number of electrons outside the nucleus of this element is _____.
2. Arrangement of electrons outside the nucleus
1. Electrons outside the nucleus are arranged in layers
2. The rules of hierarchical arrangement of electrons outside the nucleus (only the first 3 layers)
(1) First "inside" and then "outside". (Principle of lowest energy)
(2) A maximum of 2 people can be arranged on the first level, and a maximum of 8 people can be arranged on the second level;
(3) The outermost shell can hold up to 8 electrons (up to 2 electrons when there is only one electron shell);
(4) Each electron shell can accommodate up to 2n2 electrons (n represents the number of electron shells).
Knowledge expansion
⑴In the same cycle, from left to right, the number of electron shells of an atom is the same (the number of electron shells outside the nucleus is equal to the number of cycles), and the number of electrons in the outermost shell increases sequentially.
⑵In the same family, the number of electrons in the outermost shell is the same but the number of electron shells increases from top to bottom.
⑶ Atoms of the same group of elements have the same number of outermost electrons and have similar chemical properties.
think about it
①How many protons and electrons are contained in 1 H2?
②How many protons and electrons are contained in 1 H3?
③How many protons and electrons are contained in 1 H3+?
④How many protons and electrons are contained in 1 H3O+?
⑤How many protons and electrons are contained in 1 NH4+?
Knowledge training
1. If there are 2 electrons in the outermost shell of an atom of an element, then this element � �
A: It must be a non-metallic element B: It must be a metallic element
C: It must be a rare gas element D: None of the above statements are correct
2. The schematic diagram of the atomic structure of a certain element is that there are _____ protons in the nucleus of the element, _____ electron shells outside the nucleus, and _____ electrons in the outermost electron shell. It is easy to _____ (fill in " Gain" or "lose") electrons to form _____ (fill in "yin" or "yang") ions.
3. The structural diagram of a certain particle is as shown in the figure, then m=_____, n=_____, x=_____. The particle is _____ (fill in the chemical symbol of the particle)
4. The structural diagram of a certain particle is as shown in the figure. If the particle carries 2 units of negative charge, its chemical symbol is ______; if the particle carries 2 units of positive charge, its chemical symbol is ______; if the particle carries 2 units of positive charge, its chemical symbol is ______; The particle is not electrical and its chemical symbol is ______.
Keywords: teaching courseware on the mystery of the composition of matter, teaching courseware on the structure of atoms, download PPT courseware for the first volume of the ninth grade chemistry of the People's Education Press, download courseware on chemistry slides for the ninth grade, download PPT courseware on the mystery of the composition of matter, download PPT courseware on the structure of the atom, .PPT format;
For more information about the PPT courseware "The Mystery of Matter: The Structure of Atoms", please click on the "The Mystery of Matter ppt Structure of Atom" ppt tag.
"Structure of Atom" The Mystery of Material Composition PPT Courseware 6:
"Structure of Atoms" The Mystery of Material Composition PPT Courseware 6 Review Questions 1. What are the definitions of molecules and atoms? 2. Characteristics of molecules and atoms? 3. What is the essence of chemical reaction? In a chemical reaction, molecules can be divided into atoms, but atoms cannot be divided further, so other methods can...
"Structure of Atom" The Mystery of Material Composition PPT Courseware 5:
"The Structure of Atoms" The Mystery of Material Composition PPT Courseware 5 Learning Objectives 1. Understand the structure of atoms and know the microscopic composition of atoms. 2. Understand the arrangement of electrons outside the nucleus and the formation process of ions. 3. Know the concept and calculation formula of relative atomic mass.
"Structure of Atom" The Mystery of Material Composition PPT Courseware 4:
"Structure of the Atom" The Mystery of the Composition of Matter PPT Courseware 4 Observe the table (Table 4-2) and think about the following questions: 1. What are the differences between different types of atoms? Different atoms have different numbers of protons, neutrons, and electrons. 2. All atoms are composed of protons, neutrons, and electrons..
File Info
Update Time: 2024-07-03
This template belongs to Chemistry courseware People's Education Press Ninth Grade Chemistry Volume 1 industry PPT template
"Structure of Atom" The Mystery of Material Composition PPT Courseware 3 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Structure of Atom" The Mystery of Material Composition PPT Courseware 3 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Structure of Atom" The Mystery of Material Composition PPT Courseware 3, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview