| Category | Format | Size |
|---|---|---|
| People's Education Press Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
"The Composition of Water" Water in Nature PPT Courseware 10
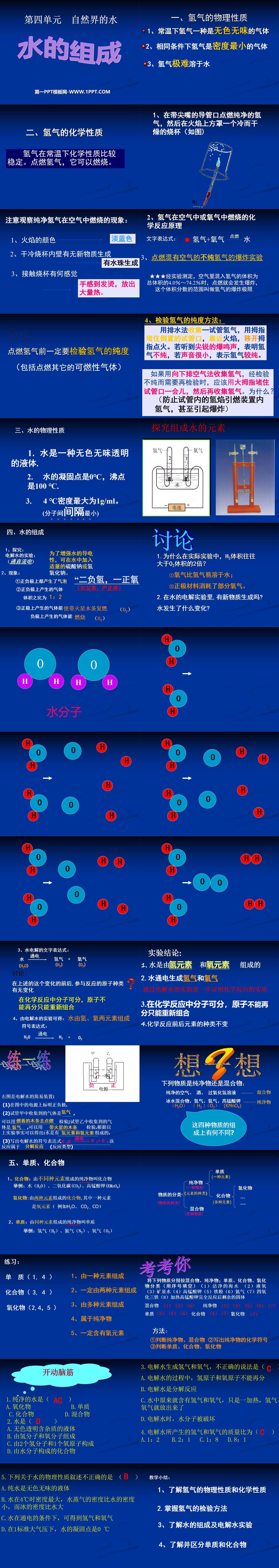
1. Physical properties of hydrogen
1. Hydrogen is a colorless and odorless gas at room temperature.
2. Hydrogen is the gas with the smallest density under the same conditions.
3. Hydrogen is extremely difficult to dissolve in water
2. Chemical Properties of Hydrogen
Hydrogen's chemical properties are relatively stable at room temperature. Ignite hydrogen and it will burn.
1. Ignite pure hydrogen at the mouth of the conduit with a pointed mouth, and then cover a cold and dry beaker over the flame (as shown in the picture)
2. The chemical reaction principle of hydrogen burning in air or oxygen
3. Explosion experiment of igniting impure hydrogen mixed with air
It has been experimentally determined that when the volume of hydrogen mixed in the air is 4.0% to 74.2% of the total volume, it will explode when ignited. This volume fraction range is called the explosion limit of hydrogen.
4. Method for testing the purity of hydrogen:
Use the drainage method to collect hydrogen in a test tube, block the mouth of the inverted test tube with your thumb, move it close to the flame, and remove your thumb to ignite it. If you hear a sharp popping sound, it means the hydrogen is impure. If the sound is very small, it means the hydrogen is purer.
If the hydrogen gas is collected using the downward air exhaust method, and it is found to be impure and needs to be re-tested, the test tube opening should be blocked with your thumb for a while, and then the hydrogen gas should be collected. Why?
(Prevent the hydrogen flame in the test tube from igniting the hydrogen in the device or even causing an explosion)
3. Physical properties of water
1. Water is a colorless, odorless and transparent liquid.
2. The freezing point of water is 0℃ and the boiling point is 100℃.
3. The maximum density at 4℃ is 1g/ml. (minimum distance between molecules)
4. Composition of water
1. Research: Experiment of electrolyzing water: (passing direct current)
In order to enhance the conductivity of water, an appropriate amount of sodium sulfate or sodium hydroxide can be added to the water.
2. Phenomenon:
① Bubbles are produced on both the positive and negative electrodes
②The ratio of the gas volumes generated on the positive and negative electrodes is 1:2
③The gas generated on the positive electrode can re-ignite the wood strips with Mars (O2)
The gas generated on the negative electrode can burn (H2)
Experimental results:
1. Water is composed of hydrogen and oxygen elements
2. Water is energized to generate hydrogen and oxygen
Further prove the essence of chemical reactions through the experiment of electrolyzing water
3. In chemical reactions, molecules can be divided, but atoms cannot be divided and can only be recombined.
4. The types of elements remain unchanged before and after the chemical reaction
5. Elements and compounds
1. Compounds: Pure substances composed of different elements are called compounds
Examples: water (H2O), carbon dioxide (CO2), potassium permanganate (KMnO4)
Oxide: A compound composed of two elements, one of which is oxygen (such as H2O, CO2, CO)
2. Elemental substance: A pure substance composed of the same element is called an elemental substance.
Examples: Hydrogen (H2), Nitrogen (N2), Oxygen (O2)
Use your brain
1.Pure water is ( )
A.Oxide B.Element
C. Compound D. Mixture
2. Water is ( )
A. Colorless, transparent liquid containing impurities
B. Composed of hydrogen molecules and oxygen molecules
C. Consists of 2 hydrogen molecules and 1 oxygen atom
D. Compounds made of water molecules
3. Electrolysis of water generates hydrogen and oxygen. The incorrect statement is ( )
A. During the process of electrolyzing water, hydrogen atoms and oxygen atoms cannot be separated again.
B. Electrolysis of water is a decomposition reaction
C. Water originally contains hydrogen and oxygen, but when it is heated, the hydrogen and oxygen are released.
D. When electrolyzing water, water molecules are destroyed
Teaching summary:
1. Understand the physical and chemical properties of hydrogen
2. Master the testing methods of hydrogen
3. Understand the composition of water and electrolysis water experiment
4. Understand and distinguish between elements and compounds
Keywords: water teaching courseware in nature, composition teaching courseware of water, PPT courseware download for the first volume of the ninth grade chemistry of the People's Education Press, chemistry slide courseware download for the ninth grade, water PPT courseware download in nature, composition of water PPT courseware download, .PPT Format;
For more information about the "Composition of Water, Water in Nature" PPT courseware, please click the "Composition of Water ppt, Water in Nature" ppt tag.
"The Composition of Water" PPT courseware:
"The Composition of Water" PPT courseware The first part of the content: Think about it. Use the knowledge you have learned and combine it with the actual life to summarize the important physical properties of water. Thinking: Water changes during its circulation in nature. Is it a physical change or a chemical change? born..
"The Composition of Water" PPT:
"The Composition of Water" PPT Part One Content: Review: 1. Judgment of physical changes and chemical changes (1) Among the following phenomena, which one is a chemical change ( ) A. Glass breakage B. Tire leakage C. Gas burning D. Air liquefaction (2) The following processes belong to...
"The Composition of Water" Source of Life - Water PPT Courseware 2:
"The Composition of Water" PPT courseware of water, the source of life 2 1. Hydrogen 1. Physical properties: colorless, odorless gas, difficult to dissolve in water, less dense than air 2. Chemical properties: flammable hydrogen Purity test and combustion Observe the experiment carefully and record the experimental phenomena to verify the purity of hydrogen..
File Info
Update Time: 2024-06-30
This template belongs to Chemistry courseware People's Education Press Ninth Grade Chemistry Volume 1 industry PPT template
"The Composition of Water" Water in Nature PPT Courseware 10 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "The Composition of Water" Water in Nature PPT Courseware 10 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"The Composition of Water" Water in Nature PPT Courseware 10, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview