People's Education Press High School Chemistry Compulsory Course I
Cantonese Education Edition Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 2
People's Education Press Ninth Grade Chemistry Volume 1
Beijing Curriculum Reform Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 1
Lu Jiao Edition Ninth Grade Chemistry Volume 1
People's Education Press Ninth Grade Chemistry Volume 2
Cantonese Education Edition Ninth Grade Chemistry Volume 2
Hunan Education Edition Ninth Grade Chemistry Volume 1
Lu Ke Edition High School Chemistry Compulsory Course 2
People's Education Press High School Chemistry Compulsory Course 2
Hunan Education Edition Ninth Grade Chemistry Volume 2
Lu Jiao Edition Ninth Grade Chemistry Volume 2
| Category | Format | Size |
|---|---|---|
| People's Education Press Ninth Grade Chemistry Volume 1 | pptx | 6 MB |
Description
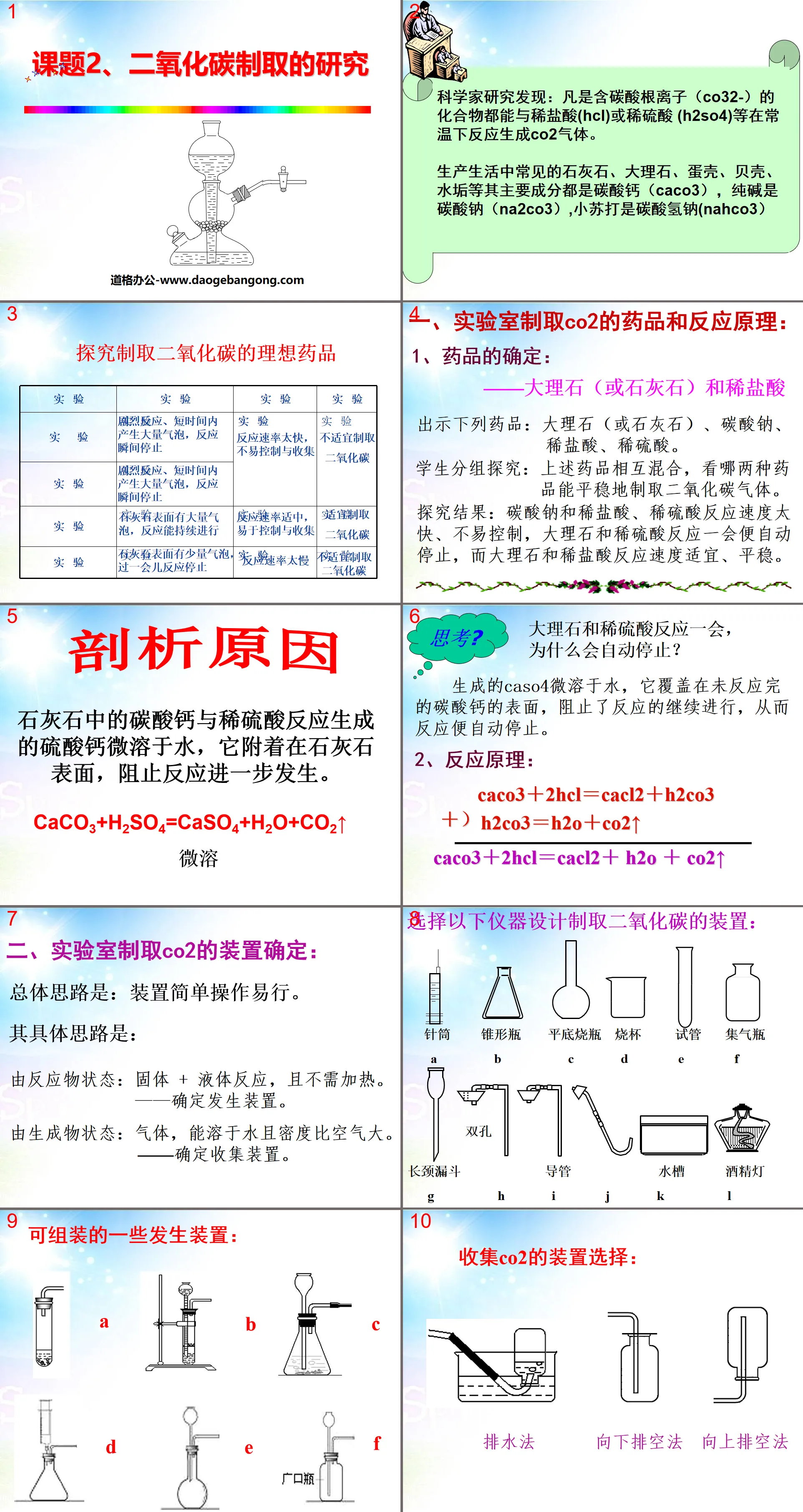
"Research on Carbon Dioxide Production" Carbon and Carbon Oxides PPT Courseware 2
Scientists have discovered that any compound containing carbonate ions (CO32-) can react with dilute hydrochloric acid (HCl) or dilute sulfuric acid (H2SO4) at room temperature to generate CO2 gas.
The main components of common limestone, marble, eggshells, shells, scale, etc. in production and life are calcium carbonate (CaCO3), soda ash is sodium carbonate (Na2CO3), and baking soda is sodium bicarbonate (NaHCO3).
1. Drugs and reaction principles for producing CO2 in the laboratory:
1. Determination of drugs: - Marble (or limestone) and dilute hydrochloric acid
Present the following medicines: marble (or limestone), sodium carbonate, dilute hydrochloric acid, dilute sulfuric acid.
Students work in groups to explore: Mix the above-mentioned drugs with each other to see which two drugs can produce carbon dioxide gas smoothly.
Research results: The reaction speed of sodium carbonate with dilute hydrochloric acid and dilute sulfuric acid is too fast and difficult to control. The reaction of marble and dilute sulfuric acid stops automatically after a while, while the reaction speed of marble and dilute hydrochloric acid is appropriate and stable.
2. Reaction principle:
CaCO3+2HCl=CaCl2+H2CO3
H2CO3=H2O+CO2↑
CaCO3+2HCl=CaCl2+H2O+CO2↑
2. Determine the device for producing CO2 in the laboratory:
The general idea is: the device is simple and easy to operate.
The specific idea is:
From the state of the reactants: solid + liquid reaction, and no heating is required. ——Determine the generating device.
The resulting product state: gas, soluble in water and denser than air. ——Determine the collection device.
You can also use the Kip generator to produce carbon dioxide gas:
Working principle: First check the air tightness of the device; then, remove the single-hole rubber stopper with the piston air guide tube, add marble from the upper side port of the container, plug the single-hole rubber stopper with the piston air guide tube and open the piston; then Add dilute hydrochloric acid from the mouth of the spherical funnel until the marble is completely submerged.
The advantage of using the Kip generator is that the reaction can occur or stop at any time, which is very convenient.
3. Steps for producing CO2 in the laboratory:
1. Check the air tightness of the device;
2. Add drugs (solid first, then liquid);
3. Collect gas.
4. Methods to verify CO2:
1. Check for fullness: Put the burning wooden stick to the mouth of the gas collection bottle. If the wooden stick goes out, it means the gas collection bottle is full.
2. Testing method: Pass the generated gas into clear lime water. If the lime water becomes turbid, it proves that the generated gas is CO2.
After studying this topic you should know
1. In the laboratory, carbon dioxide can be produced by reacting limestone (or marble) with dilute hydrochloric acid.
2. The following devices can be used in the laboratory to produce and test carbon dioxide.
3. Pay attention to the following when preparing gas in the laboratory:
(1) Select appropriate reactions, including reactants and reaction conditions;
(2) Select appropriate experimental equipment;
(3) The gas produced needs to be verified.
try your skills
1. The reason why the laboratory only uses the upward air exhaust method to collect CO2 ( )
A. Chemically inactive at room temperature
B. CO2 can be dissolved in water
C. CO2 is denser than air
D. CO2 is soluble in water and is denser than air
2. The correct way to test CO2 is ( )
A. Put the burning stick into the gas bottle and the flame will go out
B. Make people feel breathless and have headaches
C. Insert the wooden stick with sparks into the gas collecting bottle, and the wooden stick will not re-ignite.
D. Pass gas into clear lime water
2. Carbon monoxide:
Carbon monoxide and carbon dioxide only differ in their chemical formulas by one oxygen atom, but their properties are obviously different.
(1) Physical properties of carbon monoxide:
Under normal circumstances, carbon monoxide is a colorless and odorless gas; under standard conditions, its density is 1.250g/L, which is slightly lighter than air; it is difficult to dissolve in water.
Carbon monoxide is produced when carbon is not burned sufficiently.
2C+O2==2CO
1. Flammability (unlike carbon dioxide)
2. Reducibility (carbon monoxide reduces copper oxide)
Observe carefully the experiment in the picture. What phenomenon did you observe?
(1) Black copper oxide turns into red copper;
(2) Clear lime water becomes turbid.
3. Toxicity
(1) Cause of poisoning
Carbon monoxide has a stronger binding ability with hemoglobin in the blood than oxygen, causing the human body to suffocate or even die due to lack of oxygen.
(2) Prevention and control measures
When using coal stoves for heating, pay attention to ventilation. When carbon monoxide poisoning occurs, the mild person will breathe a large amount of air, and the severe person will be sent to the hospital for treatment.
2. Determine right or wrong and explain the reasons
1. Some people say that as soon as you smell the smell of gas, move the coal stove outside as soon as possible.
This statement is wrong. If you smell gas, you should first open a window for ventilation, then close the gas valve and look for leaks.
2. Some people say that as long as you put a basin of water on the coal stove, you can prevent gas poisoning.
This statement is baseless. Because CO is neither soluble in water nor reacts with water.
1. (2010 Guangxi Guilin High School Entrance Examination) Through comparison, I found that O2 and CO2 are collected in the laboratory ( )
A. Both can use the drainage and gas collection method.
B. Drainage and gas collection methods cannot be used
C. The downward air exhaust method can be used
D. Both can use the upward air exhaust method.
2. (2011 Anhui High School Entrance Examination) It is reported that chemists have created a sugar powder-like substance with a strong ability to absorb CO2 - "dry water". Each particle contains 95% water, and the outer layer is silica. Which of the following statements is correct ( )
A. Dry water and dry ice are the same substance
B. Only physical changes occur when dry water absorbs CO2
C. The molecules in dry water no longer move
D. Dry water is a mixture
Keywords: teaching courseware of carbon and carbon oxides, research teaching courseware of carbon dioxide production, download of PPT courseware for ninth grade chemistry volume 1 of People's Education Edition, download of ninth grade chemistry slide courseware, download of carbon and carbon oxide PPT courseware, carbon dioxide Download the prepared research PPT courseware in .PPT format;
For more information about the PPT courseware "Research on the Preparation of Carbon Dioxide from Carbon and Carbon Oxides", please click the Research ppt tab on Carbon and Carbon Oxides ppt Preparation of Carbon Dioxide.
"Research on Carbon Dioxide Production" Carbon and Carbon Oxides PPT Courseware 9:
"Research on Carbon Dioxide Production" Carbon and Carbon Oxides PPT Courseware 9 1. Explore the drugs and reaction principles for producing CO2: Question 1 The following four methods all produce carbon dioxide. Can they all be used to produce carbon dioxide in the laboratory? Tips: Whether the generated gas is pure,...
"Research on Carbon Dioxide Production" Carbon and Carbon Oxides PPT Courseware 8:
"Research on Carbon Dioxide Production" Carbon and Carbon Oxides PPT Courseware 8 Teaching Objectives (1) Understand the principles of carbon dioxide production in the laboratory. (2) Explore the device for producing carbon dioxide in the laboratory and use the designed device to produce carbon dioxide. (3) Understand...
"Research on Carbon Dioxide Production" Carbon and Carbon Oxides PPT Courseware 7:
"Research on Preparation of Carbon Dioxide" Carbon and Carbon Oxides PPT Courseware 7 Ask a question: What is the principle of producing carbon dioxide in the laboratory? --------What drugs are used and how to react? What matters and requirements should be paid attention to in the principle of producing gas in the laboratory? Easy to operate..
File Info
Update Time: 2024-11-22
This template belongs to Chemistry courseware People's Education Press Ninth Grade Chemistry Volume 1 industry PPT template
"Research on Carbon Dioxide Production" Carbon and Carbon Oxides PPT Courseware 2 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Research on Carbon Dioxide Production" Carbon and Carbon Oxides PPT Courseware 2 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Research on Carbon Dioxide Production" Carbon and Carbon Oxides PPT Courseware 2, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview