People's Education Edition Physics for Grade 8, Volume 2
People's Education Edition Physics for Grade 8, Volume 1
People's Education Edition Ninth Grade Physics Complete Book
Shanghai Science Edition Ninth Grade Physics
Shanghai Science Edition 8th Grade Physics
Beijing Normal University eighth grade physics volume one
Lu Jiao Edition Ninth Grade Physics Volume 2
Beijing Normal University Ninth Grade Physics Volume 1
Lu Ke Edition High School Physics Compulsory Course One
Guangdong and Shanghai Edition Ninth Grade Physics Volume 1
People's Education Press High School Physics Compulsory Course II
Lu Jiao Edition Ninth Grade Physics Volume 1
Beijing Normal University Ninth Grade Physics Volume 2
Lu Jiao Edition Eighth Grade Physics Volume 2
Lu Jiao edition eighth grade physics volume 1
Guangdong and Shanghai Edition Ninth Grade Physics Volume 2

| Category | Format | Size |
|---|---|---|
| Beijing Normal University Ninth Grade Physics Volume 1 | pptx | 6 MB |
Description
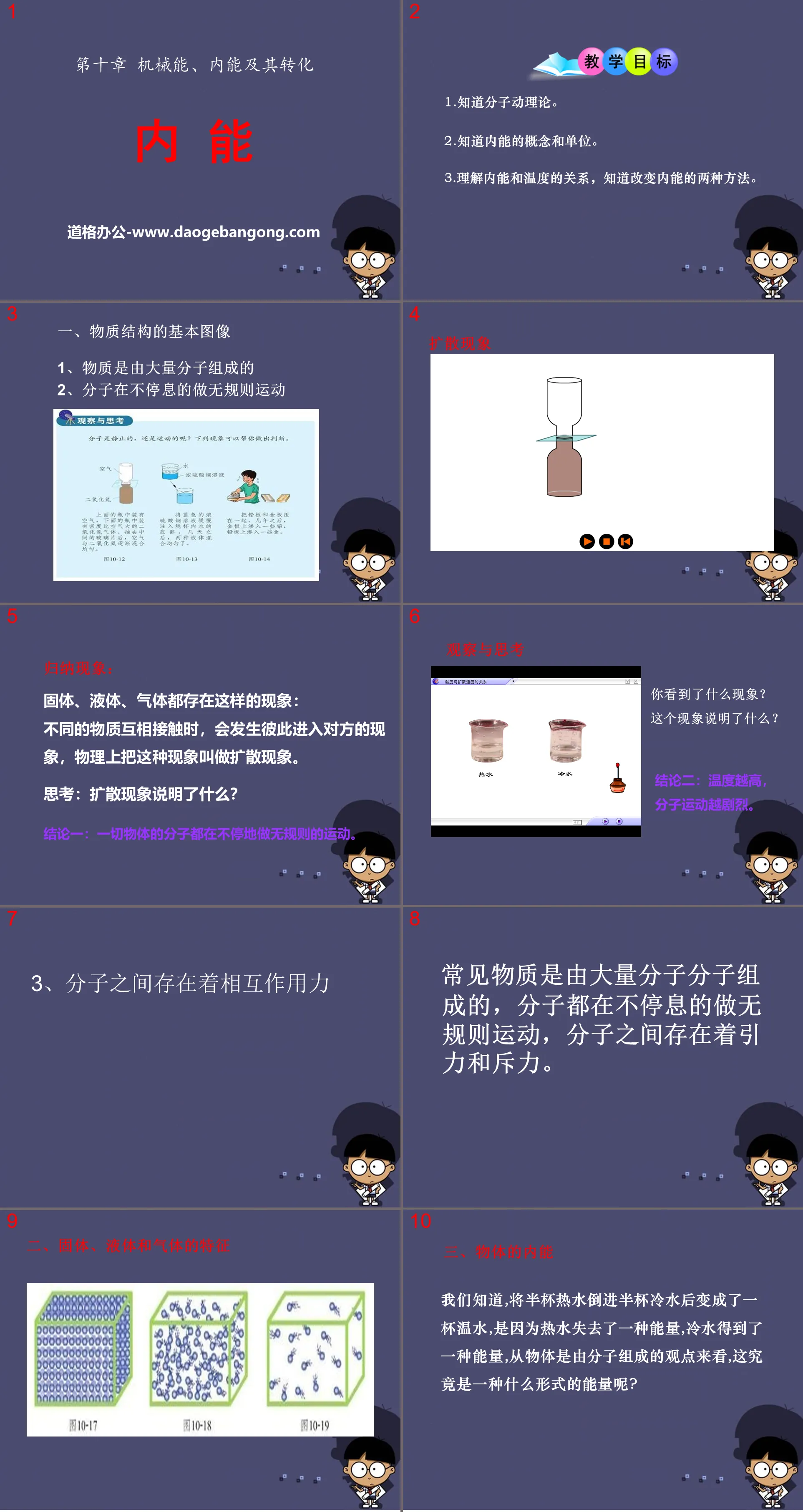
"Internal Energy" Mechanical energy, internal energy and their transformation PPT courseware 6
teaching objectives
1. Know the molecular kinetic theory.
2. Know the concept and units of internal energy.
3. Understand the relationship between internal energy and temperature, and know the two methods of changing internal energy.
1. Basic image of material structure
1. Matter is composed of a large number of molecules
2. Molecules are constantly moving irregularly
Summarize the phenomenon:
Solids, liquids, and gases all have this phenomenon: when different substances come into contact with each other, they will enter each other. In physics, this phenomenon is called diffusion.
Think: What does the diffusion phenomenon indicate?
Conclusion 1: The molecules of all objects are constantly moving irregularly.
Observe and think
What did you see?
What does this phenomenon indicate?
Conclusion 2: The higher the temperature, the more intense the molecular motion.
3. There are interactions between molecules
Common substances are composed of a large number of molecules. The molecules are constantly moving irregularly. There are attraction and repulsion forces between the molecules.
2. Characteristics of solids, liquids and gases
3. Internal energy of an object
We know that when half a cup of hot water is poured into half a cup of cold water, it becomes a cup of warm water. This is because the hot water loses an energy and the cold water gains an energy. From the perspective that objects are composed of molecules, what exactly is this? What form of energy is it?
Observe and compare:
①The moving basketball has kinetic energy, but what about the moving molecules?
Conclusion: Moving molecules also have kinetic energy.
②The apple and the earth attract each other with potential energy
Do molecules that attract each other also have potential energy?
Conclusion: Molecules that attract each other have potential energy
③ Each part of the compressed spring repels each other and has potential energy.
Discussion: Is there potential energy between molecules that repel each other?
Conclusion: Molecules that repel each other also have potential energy. Because there are interactions between molecules, there is potential energy between molecules.
Internal energy
1. Definition: The sum of the kinetic energy and molecular potential energy of a large number of molecules in an object undergoing thermal motion.
2. Thermal motion: the irregular motion of a large number of molecules inside an object.
3. The relationship between thermal motion and internal energy: the higher the temperature of the object, the more intense the thermal motion of the molecules, and the greater the internal energy.
Do the following two objects have internal energy?
The temperature is very high, the molecules move fiercely, and it has internal energy.
Although the temperature is low, the internal molecules are still moving irregularly, and it also has internal energy.
Conclusion: Any object has internal energy. Because the molecules in the object are constantly moving irregularly.
think about it
1. What happens to the internal energy of an object when its temperature increases or decreases?
As the temperature of an object increases, the internal energy increases. As the temperature of an object decreases, the internal energy decreases.
2. What is the difference between internal energy and mechanical energy?
Internal energy is energy related to microscopic motion and related to changes in temperature;
Mechanical energy is energy related to macroscopic motion, and is related to the speed and height of motion.
3. A small glass of water and a large bucket of water have the same temperature. Do they have the same internal energy? If different, which one has greater internal energy? What does it mean?
Different, there can be more in a bucket of water. It shows that the internal energy of an object is also related to the number of molecules.
How to change internal energy
Your hands get cold in winter, how do you usually keep them warm?
Both of the above methods can increase the internal energy of the hand.
①Heat transfer changes internal energy
Heat transfer can change the internal energy of an object
High-temperature objects release heat and their internal energy decreases; low-temperature objects absorb heat and their internal energy increases.
②Work changes internal energy
What examples do the ancients use to drill wood to make fire have the same principle?
Energy transformation: mechanical energy → internal energy;
Temperature changes: all are warming;
Whether to do work: to overcome friction and do work.
Doing work can change internal energy
Scientific inquiry: Doing work changes the internal energy of an object
Experiment 1, as shown in the picture, inject a small amount of ether into the thin-walled metal cylinder, plug it in, and wrap a rope around the outer wall of the metal cylinder 1-2 times. Then press the arrow in the picture
Pull the rope back and forth in the direction.
(1) What happened:
The rubber stopper will pop up and white mist will appear.
(2) In the process of pulling the rope back and forth, is there any work done?
Work against friction
(3) How is energy transformed?
mechanical energy→internal energy
(4) What changes happen to ether?
The internal energy increases, the temperature rises, and the ether vaporizes.
(5) What is the reason for the increase in internal energy of ether?
When the outside world does work on an object, the internal energy of the object increases.
Experiment 2, compression ignition nitrocellulose
(1) Why choose nitrocellulose?
Because nitrocellulose contains ether, ether has a low ignition point and is flammable.
(2) When depressing the piston, is there any work done? Who does work on whom in the device?
Work is done, the piston does work on the air
(3) How is energy transformed?
Mechanical energy → Internal energy The internal energy of the air in the cylinder increases and the temperature rises.
(4) What causes cotton to burn?
Reaching the ignition point of ether
(5) How to increase the internal energy of air?
When work is done on the air, the internal energy of the air increases
Summary of this lesson
1.Definition of internal energy
The sum of the kinetic energy and potential energy of all molecules inside an object is called the internal energy of the object.
2. Methods of changing internal energy
(1) Heat transfer (2) Work
3. Law of conservation of energy
Energy neither disappears nor is created out of thin air. It only transforms from one form to another, or transfers from one object to another. In the process of transformation and transfer, the total amount of energy constant.
Class exercises
1. The ways to change the internal energy of an object are _______ and _____.
2. The sum of __________________ and _________ inside an object is called the internal energy of the object. As the temperature of the object increases, the internal energy of the object ______.
3. In winter, breathing into your hands with your mouth will warm your hands. This is a ______ method to increase the internal energy of your hands. Rubbing your hands can also warm your hands. This is a ____ method to increase the internal energy of your hands. .
4.Judgement questions:
A The internal energy of ice at 0°C is zero ( )
B An object with zero mechanical energy must have zero internal energy ( )
C As the temperature of an object increases, the internal energy must increase ( )
D An object with a high temperature must have a large internal energy ( )
E When an object absorbs heat, its internal energy must increase ( )
5. The following process is the conversion of internal energy into mechanical energy ( )
A Use the grinding wheel to sharpen the knife. B The gas stove boils water and the water temperature rises.
C The rocket soars into the sky D The meteorite falls into the atmosphere and becomes a meteor
6. Point out how most of the energy is converted in the following phenomena?
When an electric fan works, ____ energy is converted into ______ energy
A lit candle’s ______ energy is converted into ____ energy
Rubbing your hands together ______ energy is converted into ____ energy
Incandescent lamps emit light that converts ____ energy into _____ energy
Keywords: Internal energy teaching courseware, Mechanical energy Internal energy and its transformation teaching courseware, Beijing Normal University edition ninth grade physics PPT courseware download, Ninth grade physics slide courseware download, Internal energy PPT courseware download, Mechanical energy Internal energy and its transformation PPT Courseware download, .PPT format;
For more information about the PPT courseware "Mechanical Energy Internal Energy and Its Transformation into Internal Energy", please click the Mechanical Energy Internal Energy and Its Transformation ppt Internal Energy ppt tag.
"Internal Energy of Objects" PPT courseware:
"Internal Energy of Objects" PPT courseware Part One: Learning Objectives 1. Understand the concept and units of specific heat capacity, and try to use specific heat capacity to explain relevant natural phenomena. 2. Understand the concept and unit of calorific value, and describe ways to improve fuel utilization efficiency. 3. Take a gasoline engine as an example...
"Internal Energy of Objects" PPT:
"Internal Energy of Objects" PPT Part One: Thermal Movement 1. Matter is composed of particles such as ________, ________ or ions. The particles that constitute matter never stop doing irregular ________. The __________________ inside an object is called heat transport..
"Heat Engine and Social Development" Internal Energy and Heat Engine PPT Courseware 3:
"Heat Engines and Social Development" Internal Energy and Heat Engines PPT Courseware 3 Teaching Objectives 1. Know the working principle of heat engines and energy conversion. 2. Understand the working principle and working process of gasoline engines. 3. The difference between gasoline engine and diesel engine. 4. Improve thermal engine efficiency. Test Preview 1 Understand...
File Info
Update Time: 2024-12-01
This template belongs to Physics courseware Beijing Normal University Ninth Grade Physics Volume 1 industry PPT template
"Internal Energy" Mechanical energy, internal energy and their transformation PPT courseware 6 Simple campus recruitment activity planning plan summary enterprise and institution recruitment publicity lecture PPT template is a general PPT template for business post competition provided by the manuscript PPT, simple campus recruitment activity planning plan summary enterprise and institution recruitment promotion Lecture PPT template, you can edit and modify the text and pictures in the source file by downloading the source file. If you want more exquisite business PPT templates, you can come to grid resource. Doug resource PPT, massive PPT template slide material download, we only make high-quality PPT templates!
Tips: If you open the template and feel that it is not suitable for all your needs, you can search for related content "Internal Energy" Mechanical energy, internal energy and their transformation PPT courseware 6 is enough.
How to use the Windows system template
Directly decompress the file and use it with office or wps
How to use the Mac system template
Directly decompress the file and use it Office or wps can be used
Related reading
For more detailed PPT-related tutorials and font tutorials, you can view: Click to see
How to create a high-quality technological sense PPT? 4 ways to share the bottom of the box
Notice
Do not download in WeChat, Zhihu, QQ, built-in browsers, please use mobile browsers to download! If you are a mobile phone user, please download it on your computer!
1. The manuscript PPT is only for study and reference, please delete it 24 hours after downloading.
2. If the resource involves your legitimate rights and interests, delete it immediately.
3. Contact information: service@daogebangong.com
"Internal Energy" Mechanical energy, internal energy and their transformation PPT courseware 6, due to usage restrictions, it is only for personal study and reference use. For commercial use, please go to the relevant official website for authorization.
(Personal non-commercial use refers to the use of this font to complete the display of personal works, including but not limited to the design of personal papers, resumes, etc.)
Preview